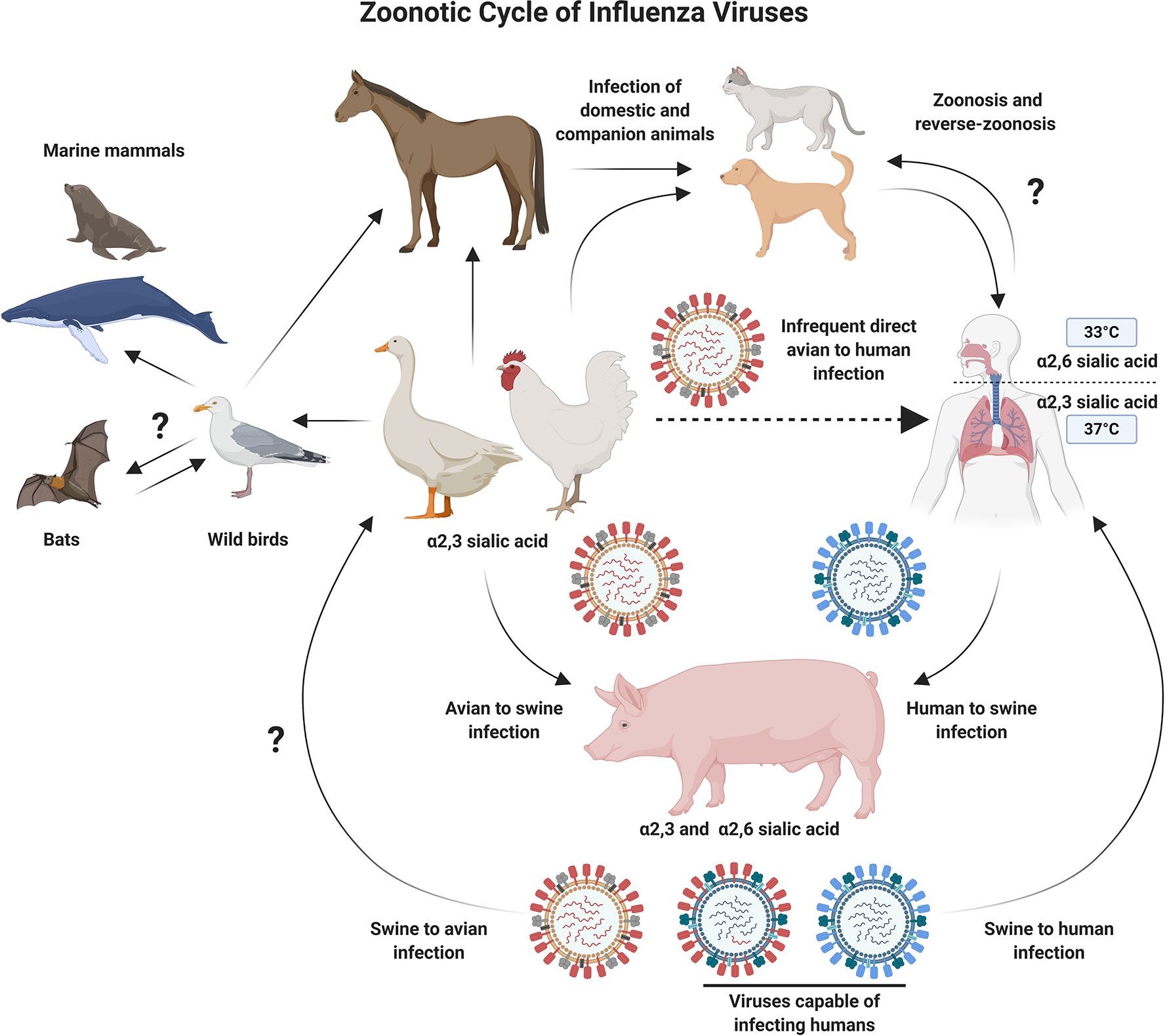
The avian flu, also known as bird flu, has been a recurring concern for global health authorities due to its potential to cause widespread illness and death in humans. The avian flu virus, particularly the H5N1 subtype, has been at the forefront of discussions regarding pandemic preparedness and response. As we move into 2024, there have been significant developments in the realm of vaccine technology and distribution, aiming to mitigate the impact of potential avian flu outbreaks. This article will delve into the latest updates on avian flu vaccines, discussing their efficacy, production, and global distribution efforts.
Understanding Avian Flu
Before diving into the specifics of vaccine updates, it’s crucial to understand the nature of the avian flu. The virus is primarily transmitted among birds, but certain strains, like H5N1, can infect humans, often with severe consequences. The primary mode of transmission to humans is through direct contact with infected birds or contaminated surfaces, rather than human-to-human transmission. This understanding underscores the importance of effective vaccination not only for human health but also as a preventative measure against the spread of the virus among poultry.
Latest Vaccine Developments
The development of effective vaccines against the avian flu has been a focal point of global health initiatives. Traditional vaccine development methods involve using inactivated viruses, which are grown in chicken eggs. However, this process can be time-consuming and may not keep pace with the rapid mutation of the flu virus. In recent years, there has been a shift towards more innovative approaches, including the use of recombinant DNA technology and mRNA-based vaccines. These newer methods allow for faster production and potentially better efficacy against a wider range of viral strains.
Recombinant DNA Technology
Recombinant DNA technology involves inserting genetic material from the flu virus into another virus or bacterium. This genetically modified organism then produces a protein that mimics part of the flu virus, which is used as the vaccine antigen. This approach enables the production of vaccines without the need for growing live viruses, enhancing safety and speeding up production times.
mRNA-Based Vaccines
mRNA (messenger RNA) vaccines represent a cutting-edge technology where a piece of genetic material (mRNA) is used to instruct cells in the body to produce a specific protein. When it comes to the avian flu, the mRNA would code for a protein found on the surface of the flu virus. The immune system recognizes this protein as foreign and mounts a response, providing immunity without exposing the individual to the actual virus. This method is not only faster and more flexible than traditional vaccine production but also potentially offers a more consistent and higher level of protection.
Global Distribution and Access
The distribution of avian flu vaccines is a complex issue, involving not only production capacity but also equitable access. Low- and middle-income countries often face challenges in accessing vaccines due to limited healthcare infrastructure, financial constraints, and geopolitical factors. International organizations such as the World Health Organization (WHO) and non-governmental organizations (NGOs) play a crucial role in facilitating access to vaccines, especially in regions where the risk of an avian flu outbreak is higher due to dense poultry farming and closer interactions between humans and birds.
Role of International Organizations
The WHO coordinates global efforts to monitor the spread of the avian flu, provides technical assistance to countries, and helps in the distribution of vaccines, especially through mechanisms like the Pandemic Influenza Preparedness (PIP) Framework. This framework aims to improve and strengthen the sharing of influenza viruses with human pandemic potential and to provide access to vaccines and sharing of other benefits.
Challenges Ahead
Despite the progress made in vaccine development and distribution, several challenges need to be addressed. The rapid mutation of the flu virus requires constant vigilance and adaptation of vaccine strains to ensure they remain effective. Additionally, the global supply chain for vaccines can be fragile, with manufacturing capacities and distribution networks subject to various risks, including political instability, economic fluctuations, and logistical hurdles.
Conclusion
The battle against the avian flu is multifaceted, involving not just the development of effective vaccines but also ensuring their equitable distribution and addressing the socio-economic factors that contribute to the risk of outbreaks. As we move forward into 2024, it’s clear that advances in vaccine technology, coupled with concerted global efforts, will be key to mitigating the impact of the avian flu. Whether through traditional methods or the latest innovations in mRNA and recombinant DNA technology, the race against time to protect human and animal health is a shared responsibility that requires collaboration, innovation, and unwavering commitment.
What are the primary modes of transmission for the avian flu to humans?
+The primary mode of transmission for the avian flu to humans is through direct contact with infected birds or contaminated surfaces, rather than human-to-human transmission.
How do mRNA-based vaccines work against the avian flu?
+mRNA-based vaccines instruct cells in the body to produce a specific protein found on the surface of the flu virus, prompting an immune response that provides immunity without exposing the individual to the actual virus.
What is the role of international organizations like the WHO in the global response to the avian flu?
+International organizations like the WHO coordinate global efforts to monitor the spread of the avian flu, provide technical assistance to countries, and facilitate access to vaccines, especially through mechanisms like the Pandemic Influenza Preparedness (PIP) Framework.