Draw 4 Water Molecules Interacting With A Li+ Ion
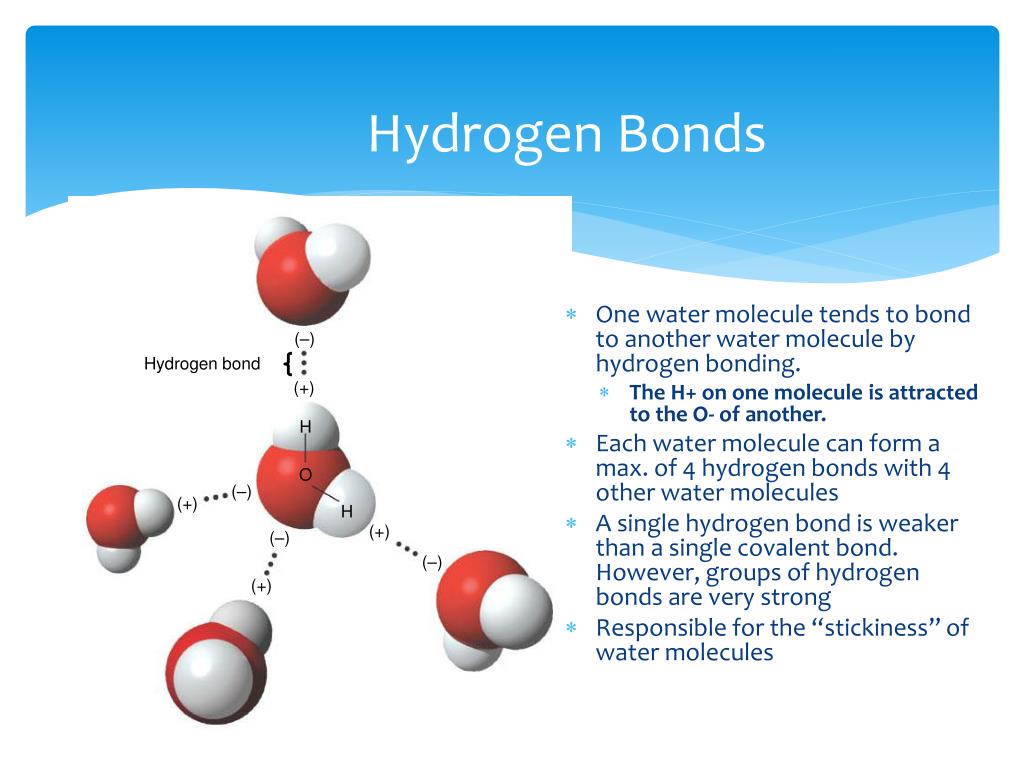
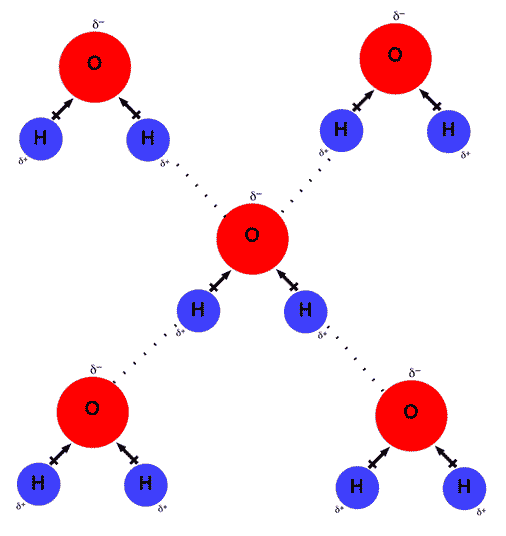
Draw 4 Water Molecules Interacting With A Li+ Ion - We have carried out molecular dynamics simulations of a li (+) ion in water over a wide range of temperature (from 248 to 368 k). Draw 4 water molecules interacting with a li+ion. Web more generally, bonds between ions, water molecules, and polar molecules are constantly forming and breaking in the watery environment of a cell. The h 2 o molecule is electrically neutral, but the positive and negative charges are not distributed uniformly. Web draw a particulate diagram of the chloride anion as it interacts with at least 4 water molecules interacting with one another.
Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. [li(oh 2)4−6]+ and [cl(h 2o)6]+. Holding 100ml of water (ebkare)________________2. (6 pts) li+ what interactions occur between two hexane molecules? Cation and anion, amount, and internuclear distance. Draw 4 water molecules interacting with a li+ion.
Intermolecular Forces Chemistry
Li+ has a smaller ionic radius than k+. For instance, a na + ion might interact with a water molecule in one. Web for example, we lithium is reacted with water in a redox reaction. Web draw a particulate diagram of the chloride anion as it interacts with at least 4 water molecules interacting.
Water molecules and their interaction with salt U.S. Geological Survey
These interactions are exothermic and bring down the energy of the system drastically. Web the cation, being positively charged, will be attracted to the negatively charged oxygen atom in the water molecule. Draw 4 water molecules interacting with a li+ion. Measuring exactly 43ml of an acid (rtube)________________4. The observed internuclear distance in the gas phase.
PPT Properties of Water PowerPoint Presentation, free download ID
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. (2pts) this question hasn't been. And thus in aqueous solution each lithium ion is associated with, i.e. Holding 100ml of water (ebkare)________________2. Which atoms are observed to be interacting with the chloride ion? Li+ has a smaller ionic radius.
Water
Holding 100ml of water (ebkare)________________2. Draw 4 water molecules interacting with a li+ion. The observed internuclear distance in the gas phase is 156 pm. Web this process, in which either a positive or a negative ion attracts water molecules to its immediate vicinity, is called hydration. You'll get a detailed solution from a subject matter.
Liquid Properties Boundless Chemistry
This is illustrated by the gradation in color in the schematic diagram here. Draw 4 water molecules interacting with a li+ion. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. All of the electron pairs—shared and unshared—repel each other. Draw 4 water.
bi·ol·o·gy (bīˈäləjē) Structure of a Water Molecule
Draw 4 water molecules interacting with a li+ion. Web calculate the amount of energy released when 1 mol of gaseous li + f − ion pairs is formed from the separated ions. Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3. [li(oh 2)4−6]+ and [cl(h 2o)6]+. Web the cation, being positively charged, will be attracted to the.
Diagram Of Water Molecule
When water molecules move closer to ions under the influence of their mutual attraction, there is a net lowering of the potential energy of the microscopic particles. Web water's large dipole moment leads to hydrogen bonding. Web value of l strongly depends on the cation type, e.g., l = 6.4, 5.1 and 1.5 for li+,.
[Solved] Draw a spacefilling model of 4 water molecules hydrogen
O o o o h h h h h h h h δ a − δ a − δ a − δ a − δ a + δ a + δ a + δ a + δ a + δ a + δ a + δ a + li+ li+ (2pts) this question hasn't been..
Types of Atoms Science at Your Doorstep
Draw 4 water molecules interacting with a li+ion. Web for example, we lithium is reacted with water in a redox reaction. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web calculate the amount of energy released when 1 mol of gaseous li + f − ion pairs.
Properties of Water Wyzant Resources
Web where licl(aq) specifies the aquated ions, i.e. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. And thus in aqueous solution each lithium ion is associated with, i.e. Draw 4 water molecules interacting with a li+ion. Besides hydrogen bonds, what other intermolecular forces.
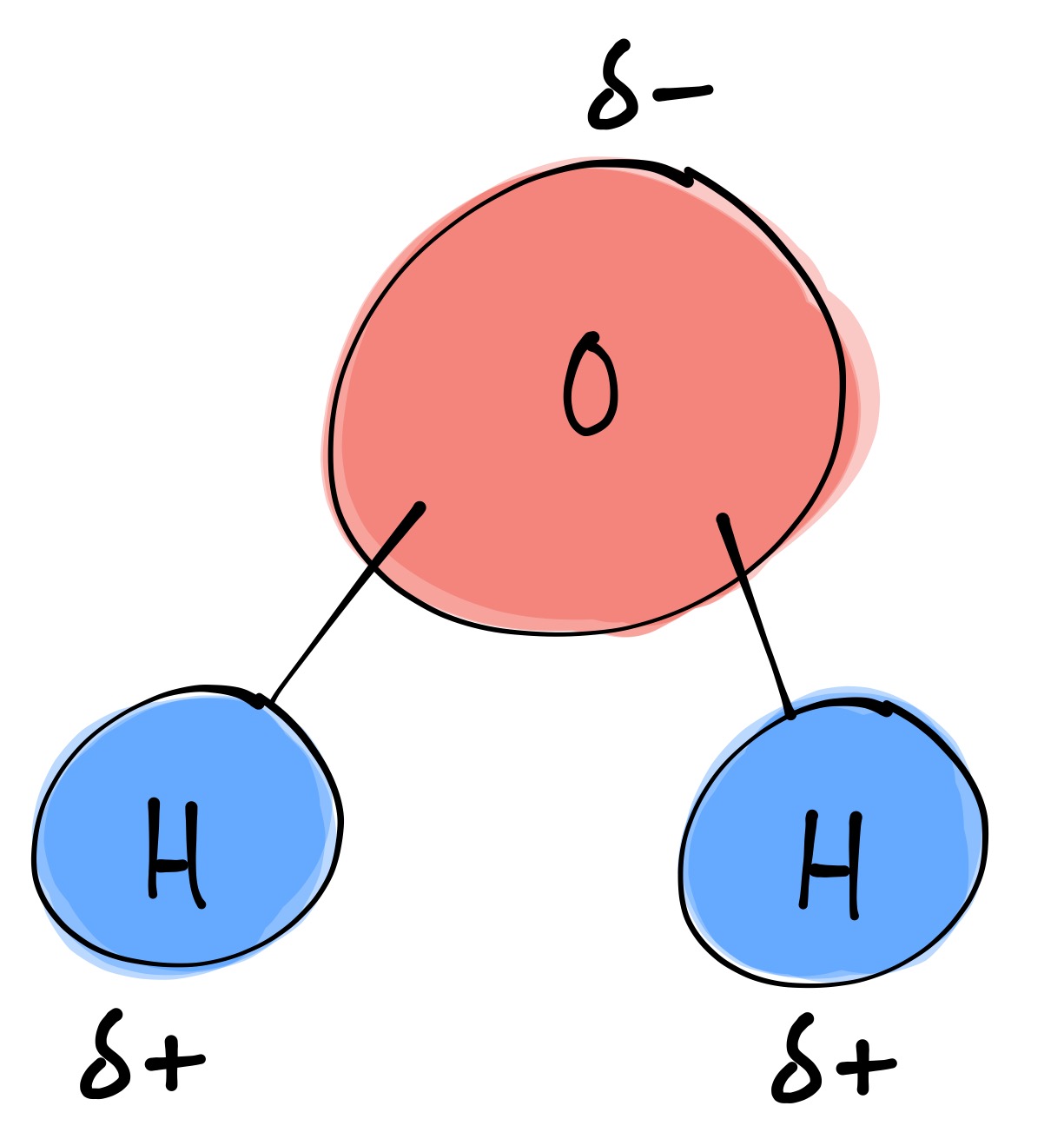
Draw 4 Water Molecules Interacting With A Li+ Ion The electronic (negative) charge is concentrated at the oxygen end of the molecule, owing. Web for example, we lithium is reacted with water in a redox reaction. [li(oh 2)4−6]+ and [cl(h 2o)6]+. Energy released from formation of gaseous ion pairs. Web value of l strongly depends on the cation type, e.g., l = 6.4, 5.1 and 1.5 for li+, na+ and cs+ ionic forms, respectively.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Draw 4 water molecules interacting with a li+ion. The h 2 o molecule is electrically neutral, but the positive and negative charges are not distributed uniformly. (2pts) this problem has been solved! When water molecules move closer to ions under the influence of their mutual attraction, there is a net lowering of the potential energy of the microscopic particles.
Web For Example, We Lithium Is Reacted With Water In A Redox Reaction.
Li+ has a smaller ionic radius than k+. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web the cation, being positively charged, will be attracted to the negatively charged oxygen atom in the water molecule. Web water's large dipole moment leads to hydrogen bonding.
O O O O H H H H H H H H Δ A − Δ A − Δ A − Δ A − Δ A + Δ A + Δ A + Δ A + Δ A + Δ A + Δ A + Δ A + Li+ Li+
Web this process, in which either a positive or a negative ion attracts water molecules to its immediate vicinity, is called hydration. Please answer as soon as possible Web draw a particulate diagram of the chloride anion as it interacts with at least 4 water molecules interacting with one another. Which atoms are observed to be interacting with the chloride ion?
Energy Released From Formation Of Gaseous Ion Pairs.
Web calculate the amount of energy released when 1 mol of gaseous li + f − ion pairs is formed from the separated ions. Draw 4 water molecules interacting with a li+ion. The electronic (negative) charge is concentrated at the oxygen end of the molecule, owing. Web draw a particulate diagram of the chloride anion as it interacts with at least 4 water molecules interacting with the chloride ion.