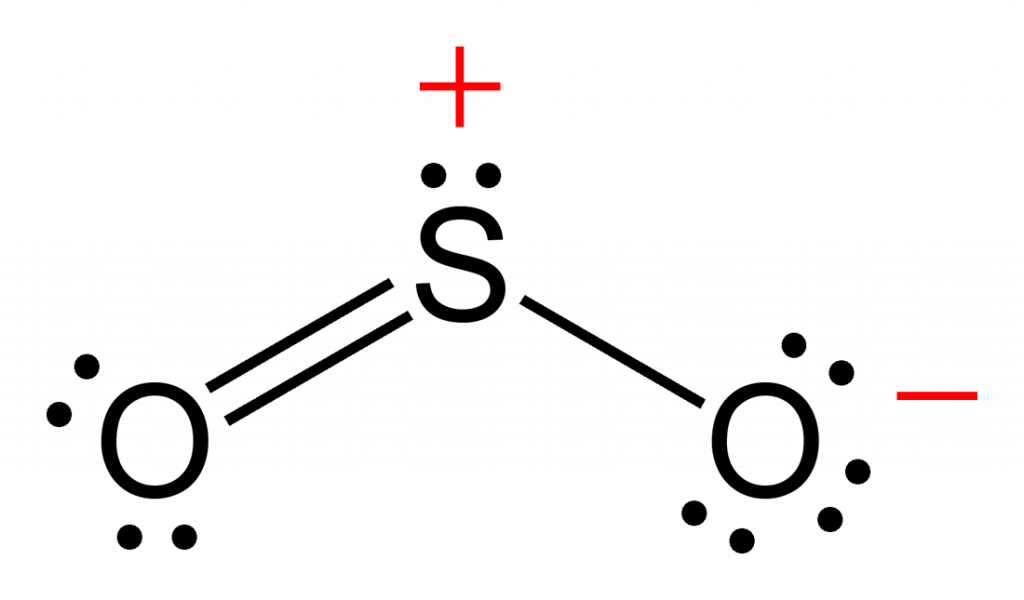
Draw All Resonance Structures For The Sulfur Dioxide Molecule So2
Draw All Resonance Structures For The Sulfur Dioxide Molecule So2 - (valence electrons are the number of electrons present in the outermost shell of an atom). Sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. Determine the total valence electrons. O double bond s double bond o with 3 the There is also a lone pair attached to the sulfur atom.
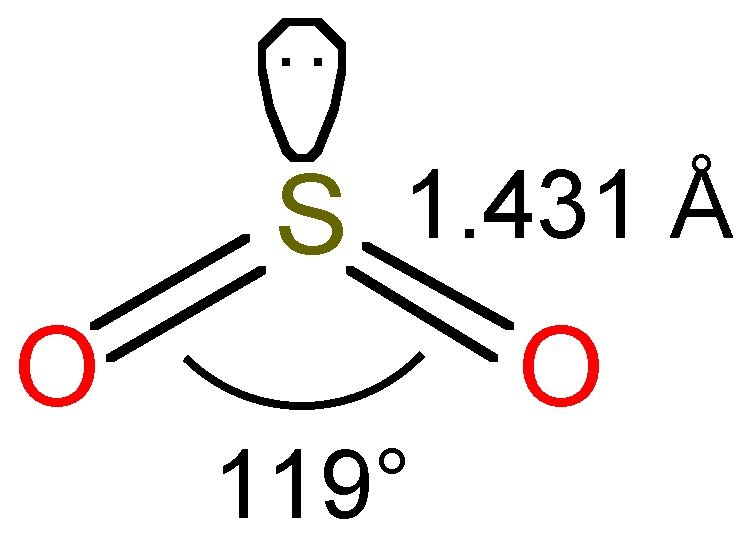
This indicated a bent molecular shape. Web chemistry chemistry questions and answers draw all resonance structures for the sulfur dioxide molecule, so2 this problem has been solved! After obtaining the lewis structure of so 2, we can determine the hybridization of atoms. Draw all resonance structures for the sulfur dioxide molecule, so2 explicitly draw all h atoms • include all valence lone pairs in your answer do not include overall ion charges or formal charges in your drawing do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule draw one. Draw one structure per sketcher. Yeah, so let's start off by counting its valence electrons. (valence electrons are the number of electrons present in the outermost shell of an atom).
Lewis Structure of Sulphur Dioxide SO2 YouTube
We start with a valid lewis structure and then follow these general rules. • include all valence lone pairs in your answer. There is also a lone pair attached to the sulfur atom. Start by counting the valence electrons of each atom in the molecule. Vsepr for 4 electron clouds. Web to determine the molecular.
SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
There is also a lone pair attached to the sulfur atom. Let's draw the first two lewis structures for so_2. • include all valence lone pairs in your answer. Do not include overall ion charges or formal charges in your drawing. So today we'll be looking at sulphur dioxide and drawing its residence forms. Web.
SO2(Sulfur Dioxide) Lewis Structure, Hybridization, Molecular Geometry
Draw one structure per sketcher box, and separate added sketcher boxes with the → symbol. Start by counting the valence electrons of each atom in the molecule. Web so the resonance structure on the left, and the resonance structure on the right, and some people disagreed with me, and said that's not the dot structure.
How to draw SO2 Lewis Structure? Science Education and Tutorials
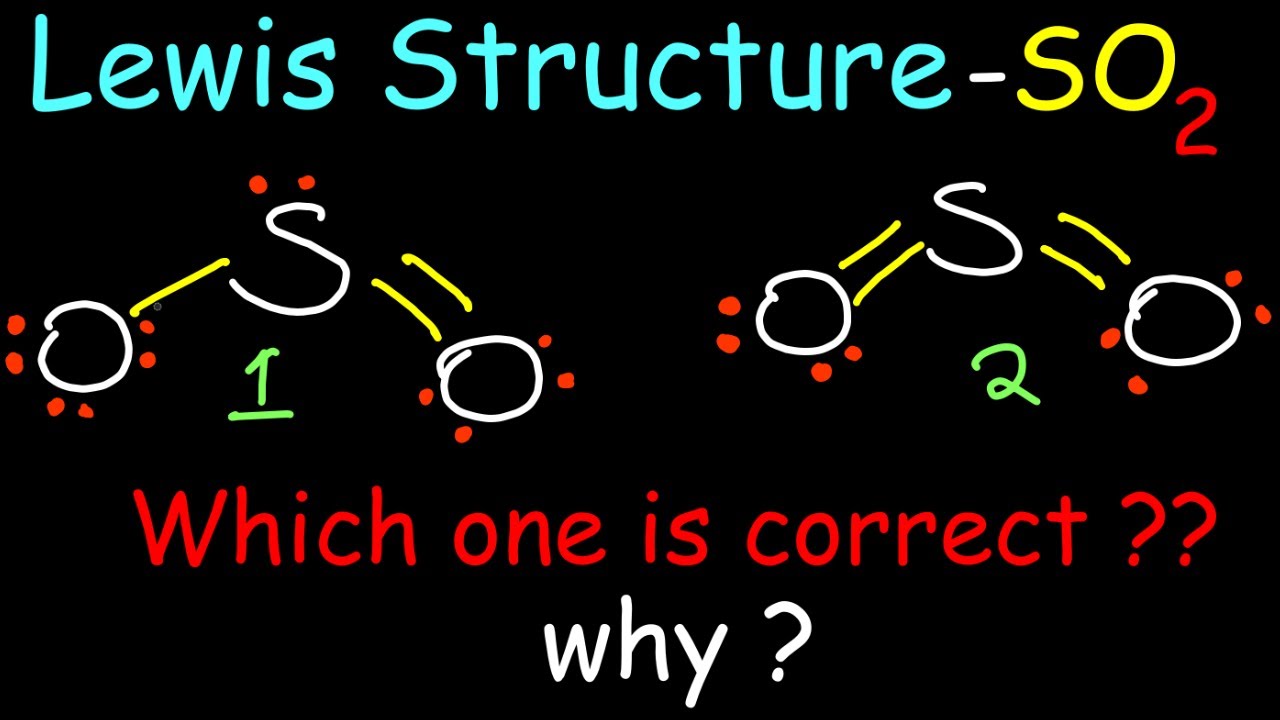
So i would assume that the one with two double bonds is the correct structure. Web sulfur dioxide, or so_2, has two resonance structures which contribute equally to the overall hybrid structure of the molecule. Web so the resonance structure on the left, and the resonance structure on the right, and some people disagreed with.
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of
Let's draw the first two lewis structures for so_2. But chemistry books i have looked at (zumdahl edition 5 and 7) says that it is the opposite. Draw all equivalent resonance structures in which the s atom obeys the octet rule for the sulfur dioxide molecule, so2. Vsepr for 3 electron clouds. Web there are.
SO2 Lewis Structure ,Valence Electrons ,Formal Charge Lewis Structure
So today we'll be looking at sulphur dioxide and drawing its residence forms. The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double. We start with a valid lewis structure and then.
SO2 Lewis Structure How to Draw the Lewis Structure for SO2 (Sulfur
Web to draw the so2 lewis structure, follow these simple steps: Let's draw the first two lewis structures for so_2. So i would assume that the one with two double bonds is the correct structure. Web draw all resonance structures for the sulfur dioxide molecule, so_2. After obtaining the lewis structure of so 2, we.
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of
Vsepr for 2 electron clouds. Determine the total valence electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Vsepr for 3 electron clouds. We start with a valid lewis structure and then follow these general rules. But chemistry books i have looked at (zumdahl edition 5 and.
Resonance Structures for SO2 (Sulfur dioxide) YouTube
Determine the total valence electrons. But chemistry books i have looked at (zumdahl edition 5 and 7) says that it is the opposite. Draw one structure per sketcher. More on the dot structure for sulfur dioxide. Do not include overall ion charges or formal charges in your drawing. Start by counting the valence electrons of.
SO2 Lewis Structure Sulfur Dioxide YouTube
Draw all equivalent resonance structures in which the s atom obeys the octet rule for the sulfur dioxide molecule, so2. Do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule. Web there are three resonance structures so2 (sulfur dioxide). Web to determine the molecular geometry.
Draw All Resonance Structures For The Sulfur Dioxide Molecule So2 Include all valence lone pairs in your answer. There is also a lone pair attached to the sulfur atom. Draw all equivalent resonance structures in which the s atom obeys the octet rule for the sulfur dioxide molecule, so2. Sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Web Resonance And Formal Charge.
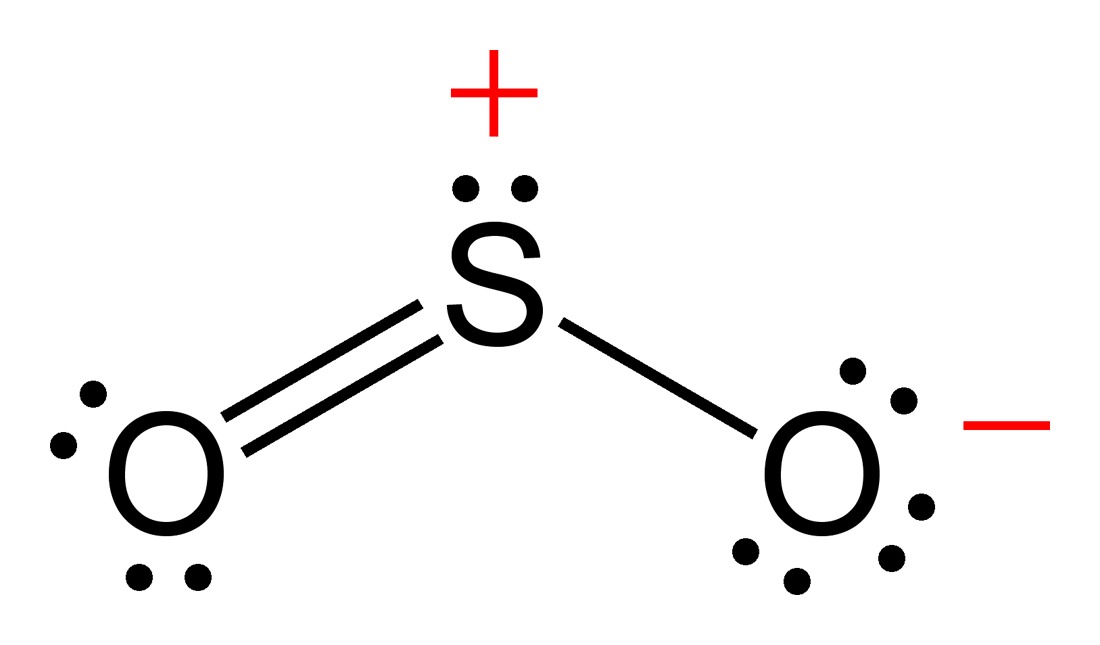
We start with a valid lewis structure and then follow these general rules. There is also a lone pair attached to the sulfur atom. Web lewis structure of o2. Web the formal charges of the so 2 with the single bond and a double bond is larger than the so 2 with two double bonds.
Do Not Show Ion Charges In Your Drawing.
(valence electrons are the number of electrons present in the outermost shell of an atom). So i would assume that the one with two double bonds is the correct structure. After obtaining the lewis structure of so 2, we can determine the hybridization of atoms. Web sulfur dioxide, or so_2, has two resonance structures which contribute equally to the overall hybrid structure of the molecule.
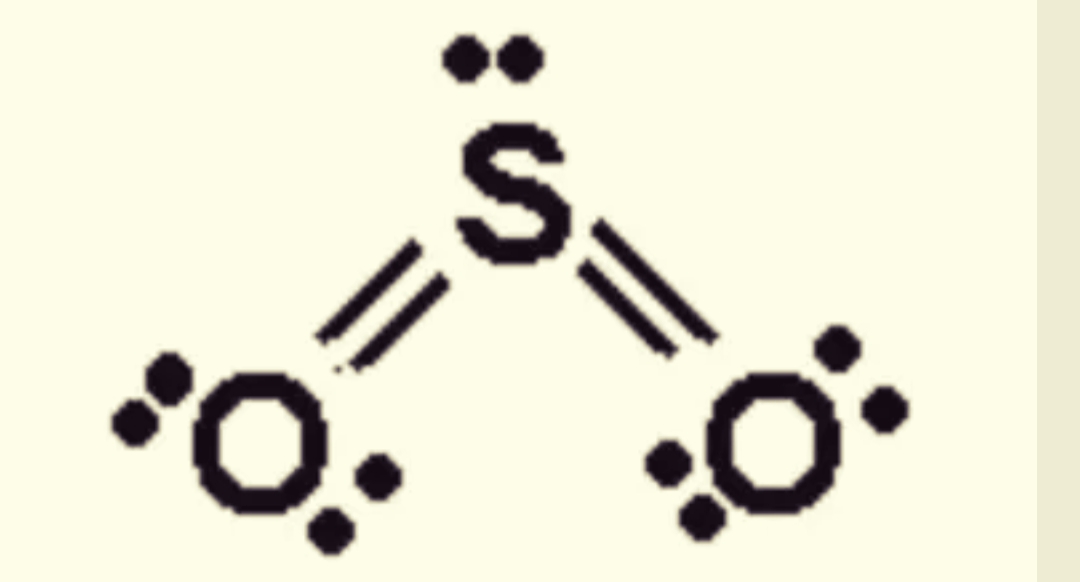
The Dot Structure For Sulfur Dioxide Has Sulfur With A Double Bond To An Oxygen On The Left, And Two Lone Pairs Of Electrons On That Oxygen, And The Sulfur With A Double.
But chemistry books i have looked at (zumdahl edition 5 and 7) says that it is the opposite. Web chemistry chemistry questions and answers draw the lewis structure for the sulfur dioxide (so2) molecule. Here, the given molecule is so2 (sulfur dioxide). Which is the correct lewis structure?
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Draw one structure per sketcher box, and separate added sketcher boxes with the → symbol. In so2, sulfur is in group 6, so it has 6 valence electrons, while each oxygen atom in group 6 contributes 6 valence electrons. Add atom symbol , i ←→ 2 this problem has been solved! Web draw all resonance structures for the sulfur dioxide molecule, so_2.