Draw Bohr Model
Draw Bohr Model - Describe the arrangement of electrons using the shell model. Bohr's model calculated the following energies for an electron in the shell, n : The boron atom along with its atomic number, electronic configuration, and atomic mass is represented as follows in the periodic table: Learn how to draw a bohr model with help from an artist in this free. Web this chemistry tutorial video walks you through how to draw a bohr model, bohr diagram, or planetary model for an atom or ion.
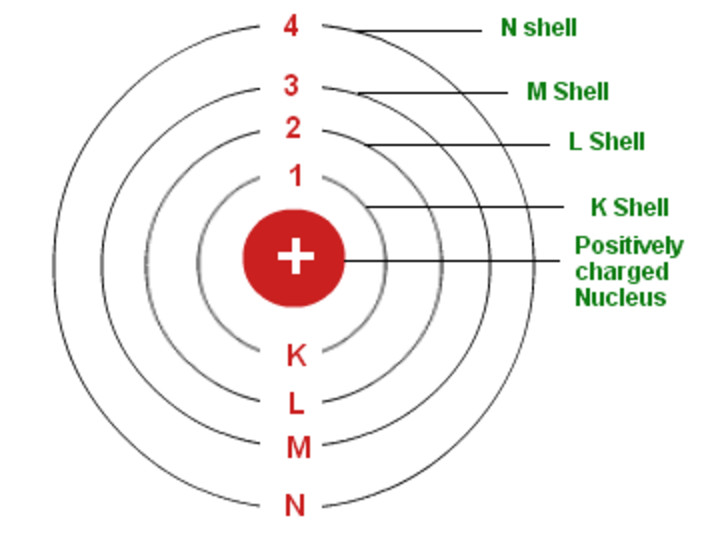
Web i'll teach you how to figure out the number of electron energy levels and the electrons that fit in each using the periodic table. Web part of the series: Write the number of protons and neutrons in the nucleus 3. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Questions tips & thanks want to join the conversation? The steps to drawing bohr diagrams for elements and atoms are. Two in the first shell, eight in the second shell, eight in the third shell.
Carbon Bohr Model — Diagram, Steps to Draw Techiescientist
The bohr model has an atom consisting of a small, positively charged nucleus orbited by negatively charged electrons. Draw shells around the nucleus to represent the orbitals. • the atomic number of boron is 5. The number of shells that you draw will depend on the number of electrons in the atom. Write the number.
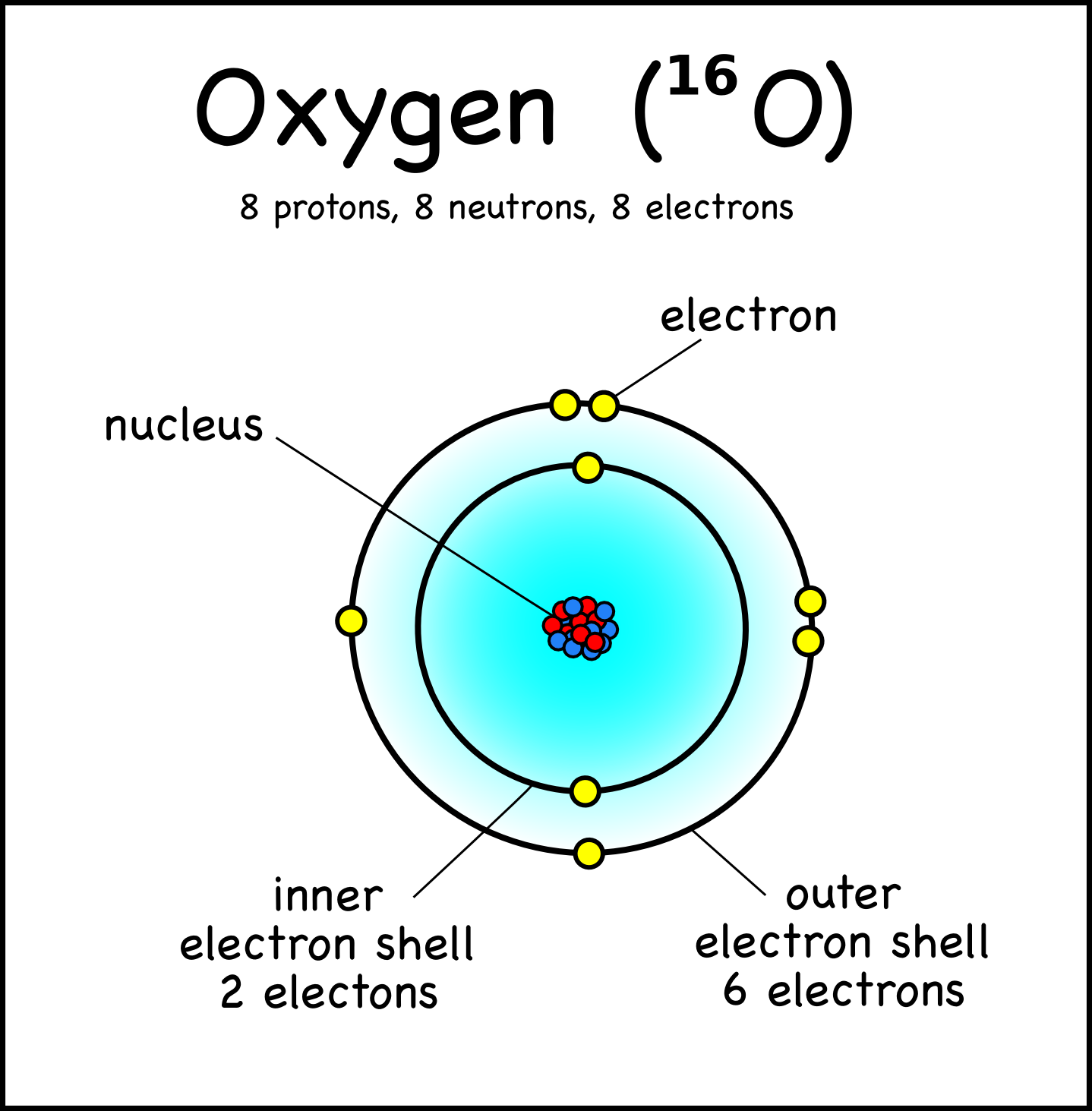
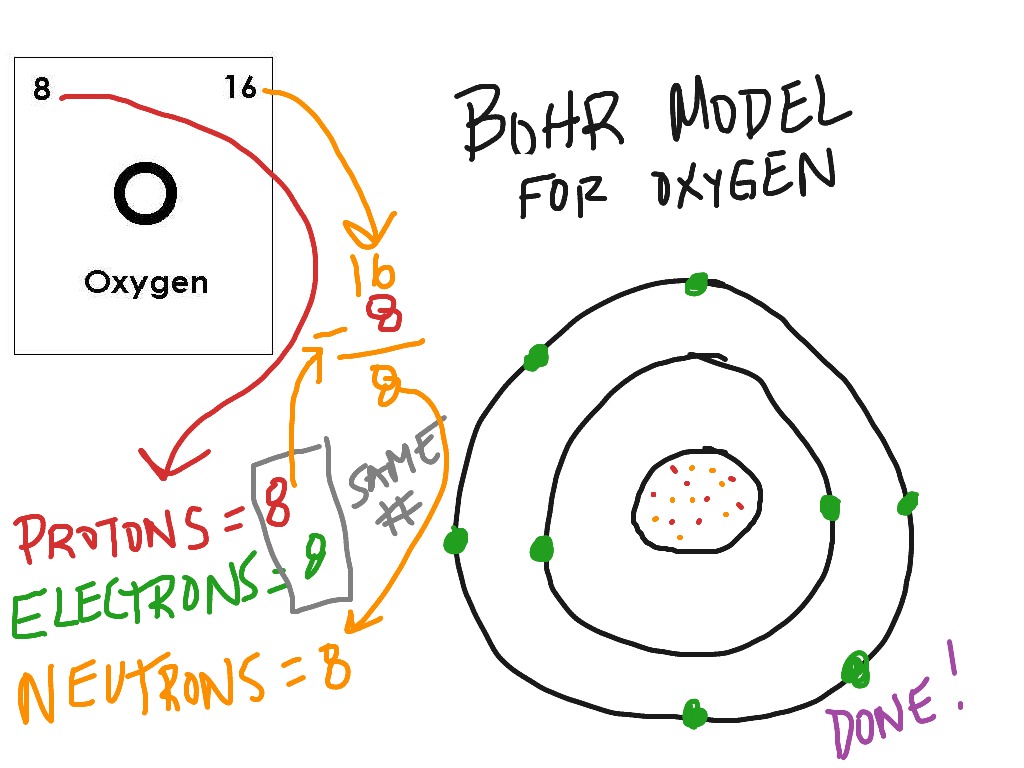
Bohr Model Drawing Of Oxygen at GetDrawings Free download
Web steps to draw bohr model of boron. Information derived from the boron box: Web this chemistry tutorial video walks you through how to draw a bohr model, bohr diagram, or planetary model for an atom or ion. Web i'll teach you how to figure out the number of electron energy levels and the electrons.
Bohr's Model of an Atom Chemistry, Class 11, Structure of Atom
Web the bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Draw the next shell if you have more electrons to add 6. Bohr described the hydrogen.
Describe Bohr’s model of the hydrogen atom. bitWise Academy
The protons and neutrons are placed into the nucleus and the. Questions tips & thanks want to join the conversation? The number of shells that you draw will depend on the number of electrons in the atom. Web the bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell.
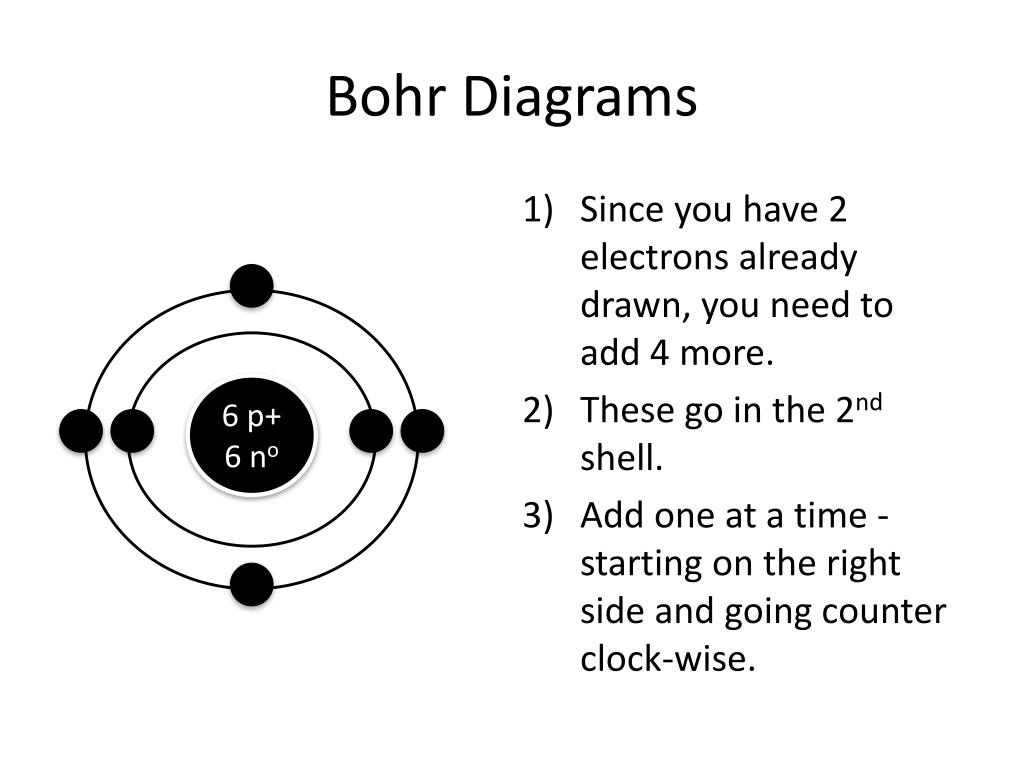
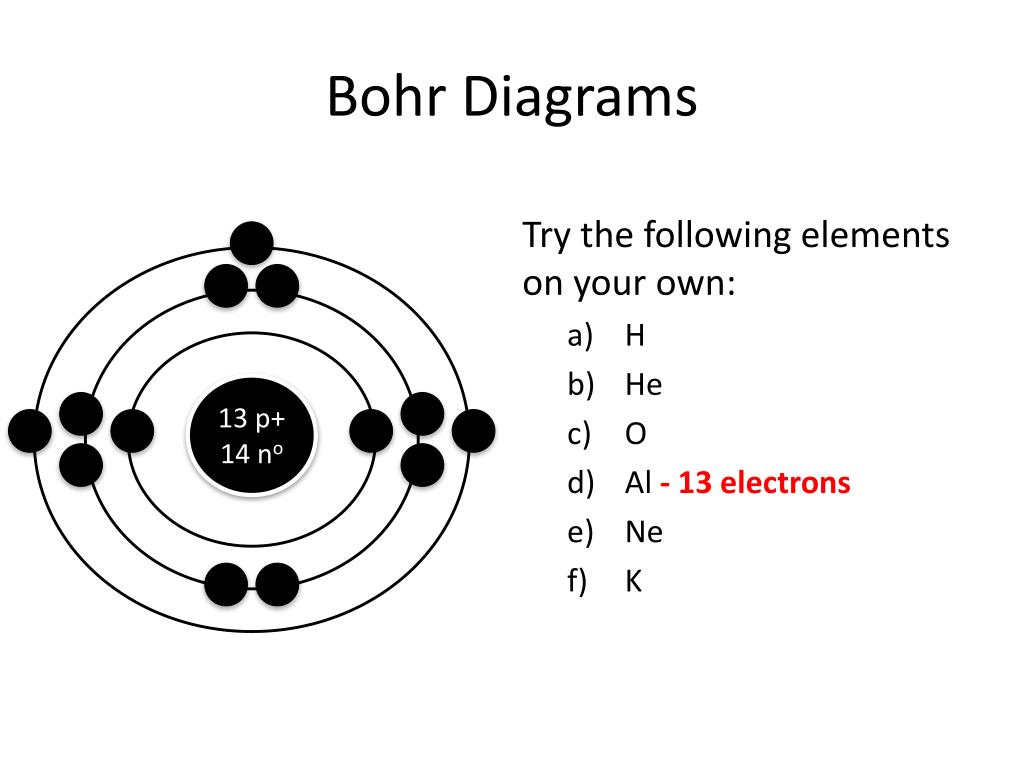
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Its value is obtained by setting n = 1 in equation 6.38: Draw the first electron shell and put the electrons as a dot in it. The number of shells that you draw will depend on the number.
Bohr Model Drawing Of Oxygen at GetDrawings Free download
Web in the bohr model, there are a few rules that will help you draw accurate diagrams. The radius of the first bohr orbit is called the bohr radius of hydrogen, denoted as a 0. Write the number of protons and neutrons at the center of the nucleus. Draw the next shell if you have.
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download
The boron family is located in the 13 th group of the periodic table: Web updated on january 27, 2020. Web the bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. You will also learn how to write bohr model electron configuration as. Web.
Bohr Model of the Atom Overview and Examples
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Web i'll teach you how to figure out the number of electron energy levels and the electrons that fit in each using the periodic table. Web the bohr model of the hydrogen atom explains the connection between the.
Potassium Bohr Model Diagram, Steps To Draw Techiescientist
Watch this video of your professor drawing bohr models and identifying valence electrons. Electrons must occupy the lowest available shell, closest to the nucleus. Write the number of protons and neutrons in the nucleus 3. Draw shells around the nucleus to represent the orbitals. He postulated that the electron was restricted to certain orbits characterized.
how to draw a bohr model
Electrons must occupy the lowest available shell, closest to the nucleus. Draw the next shell if you have more electrons to add 6. Niels bohr proposed an early model of the atom as a central nucleus containing protons and neutrons. The radius of the first bohr orbit is called the bohr radius of hydrogen, denoted.
Draw Bohr Model Draw the electrons in their respective orbitals. Draw the first electron shell and put the electrons as a dot in it. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Learn how to draw a bohr model with help from an artist in this free. Two in the first shell, eight in the second shell, eight in the third shell.
Draw Shells Around The Nucleus To Represent The Orbitals.
Draw the second electron shell,. Web drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. Questions tips & thanks want to join the conversation? Draw a circle to represent the nucleus of the atom.
Add Your Electrons, The 2Nd Shell Can Hold Up To 8 P:9 N:10 Be Sure To Add Them One At A Time Going Clock Wise.
Web i'll teach you how to figure out the number of electron energy levels and the electrons that fit in each using the periodic table. Draw the electrons in their respective orbitals. This process will allow us to identify the valence or active electrons of these particular elements. Draw the first electron shell and put the electrons as a dot in it.
Electrons Must Occupy The Lowest Available Shell, Closest To The Nucleus.
Web updated on january 27, 2020. Information derived from the boron box: A 0 = 4 π ε 0 ℏ 2 m e e 2 = 5.29 × 10 −11 m = 0.529 å. Top voted ali arshad 9 years ago what does the negative sign in energy term represent ?
Its Value Is Obtained By Setting N = 1 In Equation 6.38:
Web the bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Watch this video of your professor drawing bohr models and identifying valence electrons. E ( n) = − 1 n 2 ⋅ 13.6 ev We’ll use a bohr diagram to visually represent where the electrons are around the nucleus of the he atom.