Draw The Electron Configuration For A Neutral Atom Of Boron.
Draw The Electron Configuration For A Neutral Atom Of Boron. - Draw the electron configuration for a neutral atom of boron. Web draw the electron configuration for a neutral atom of boron. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Should the sixth electron be placed in the same 2 p orbital that already has an electron, or should it go in one of the empty 2 p orbitals? Web oct 1, 2016 the noble gas configuration is [he]2s^22p^1.
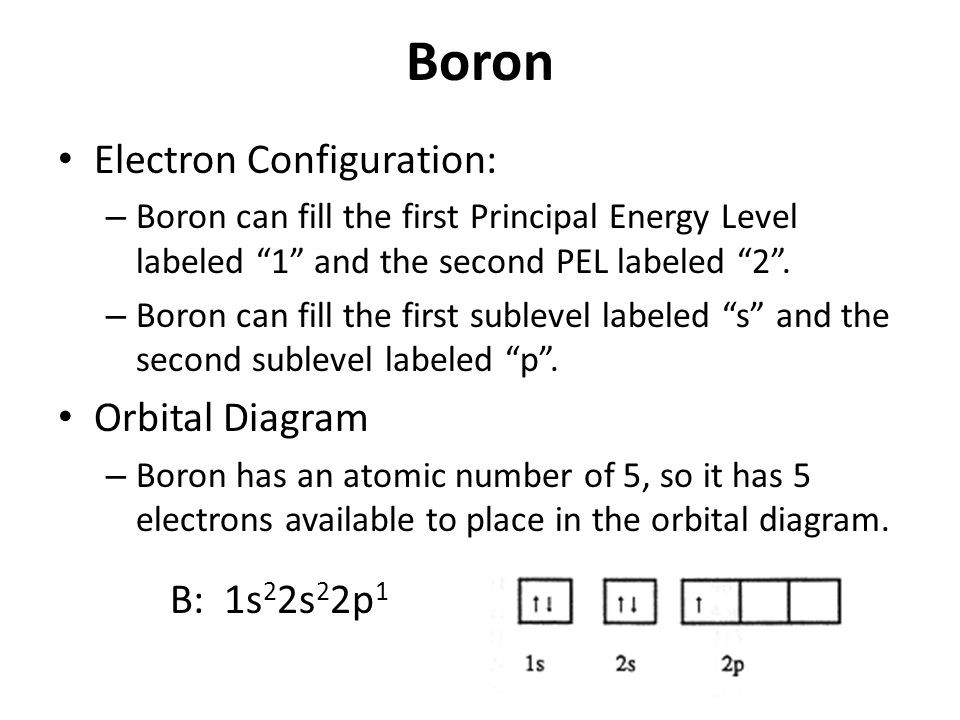
Web draw the electron configuration for a neutral atom of boron. Web the electron configuration of boron is 1s 2 2s 2 2p 1: Thus, it is simple to determine the charge on such a negative ion: Web the arrangement of electrons in boron in specific rules in different orbits and orbitals is called the electron configuration of boron. Electron configuration through orbit (bohr principle) Electron configuration of carbon (c) [he] 2s 2 2p 2: Draw the electron configuration for a neutral atom of nickel.
Electron arrangements
Web its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used: Draw the electron configuration for a neutral atom of iron. Draw the electron configuration for a neutral atom of nickel. Web the arrangement of electrons in boron in specific rules in different orbits and orbitals is called the electron.
Boron Electron Configuration And Full Orbital Diagram
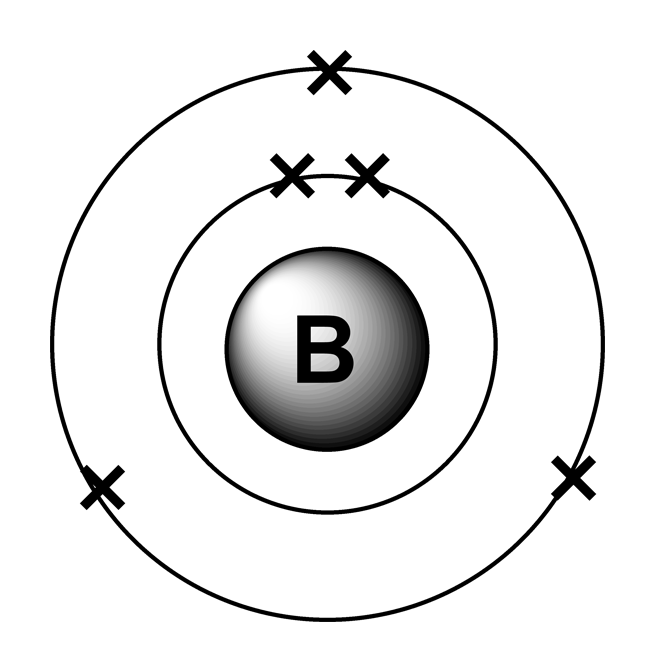
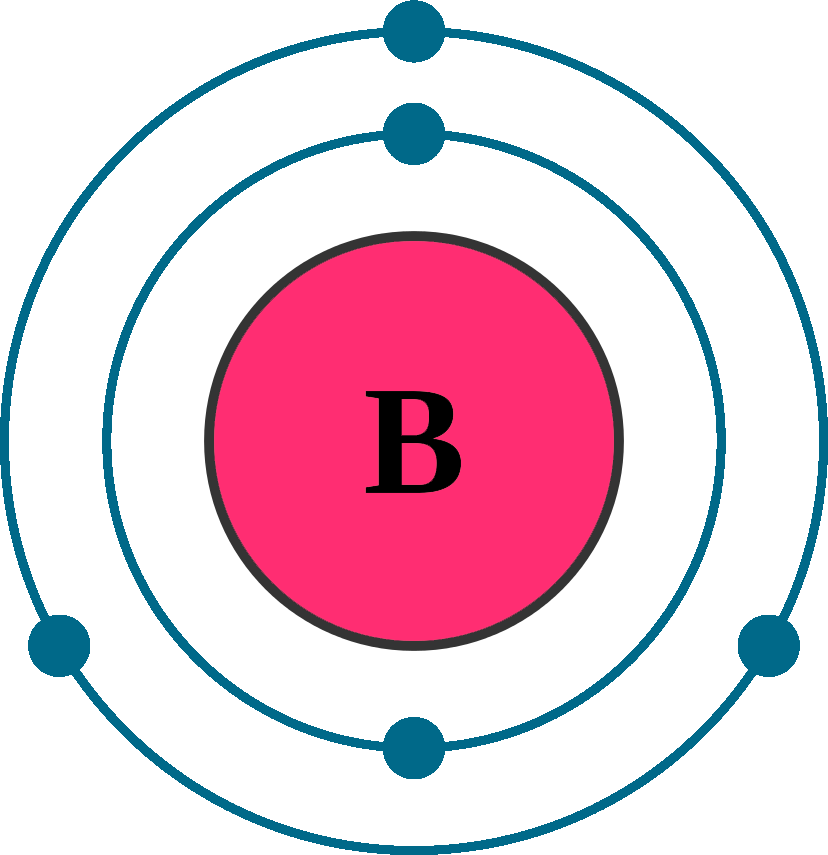
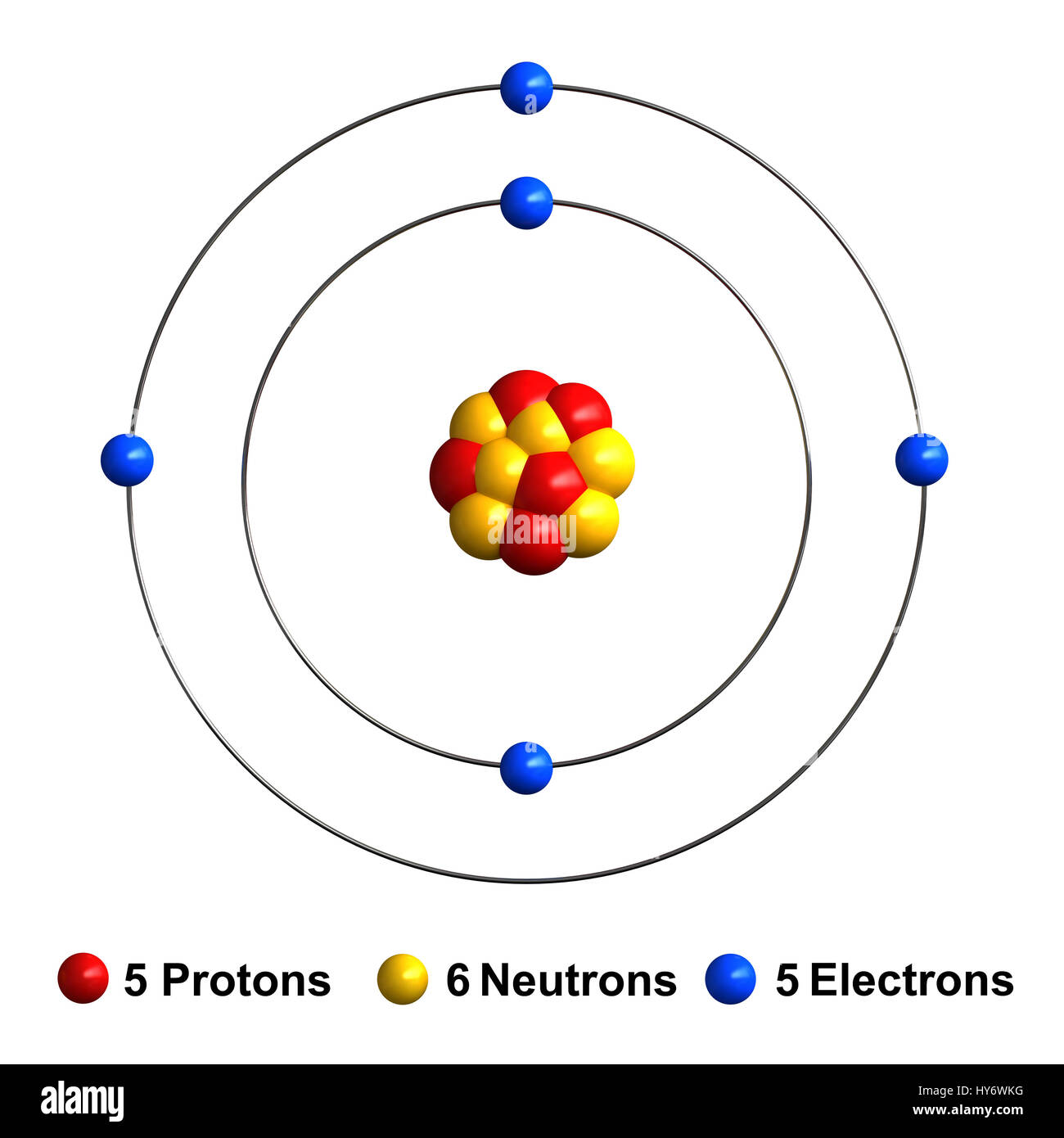
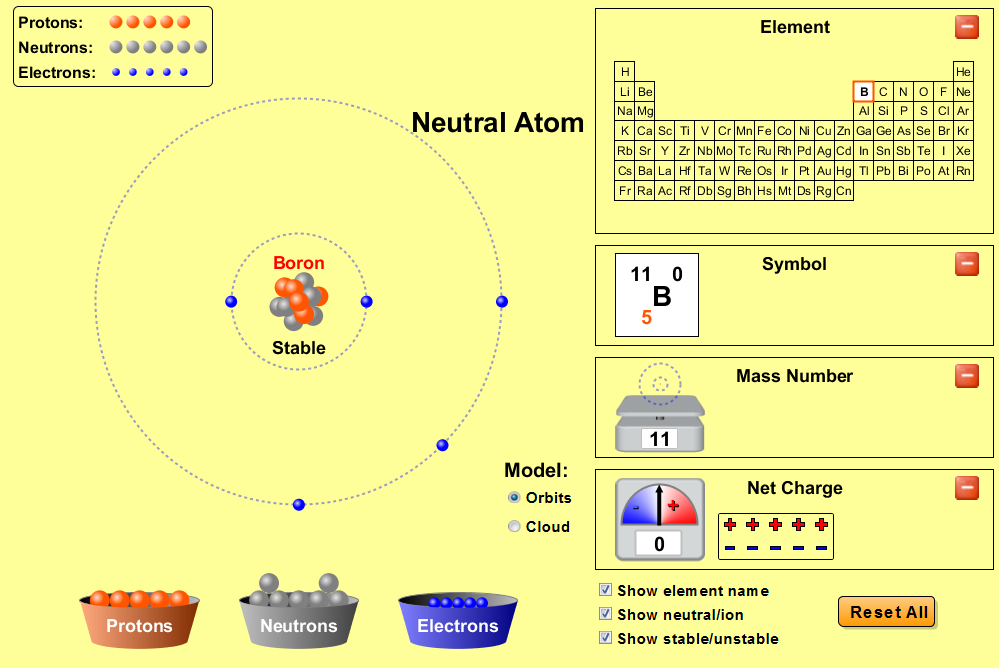
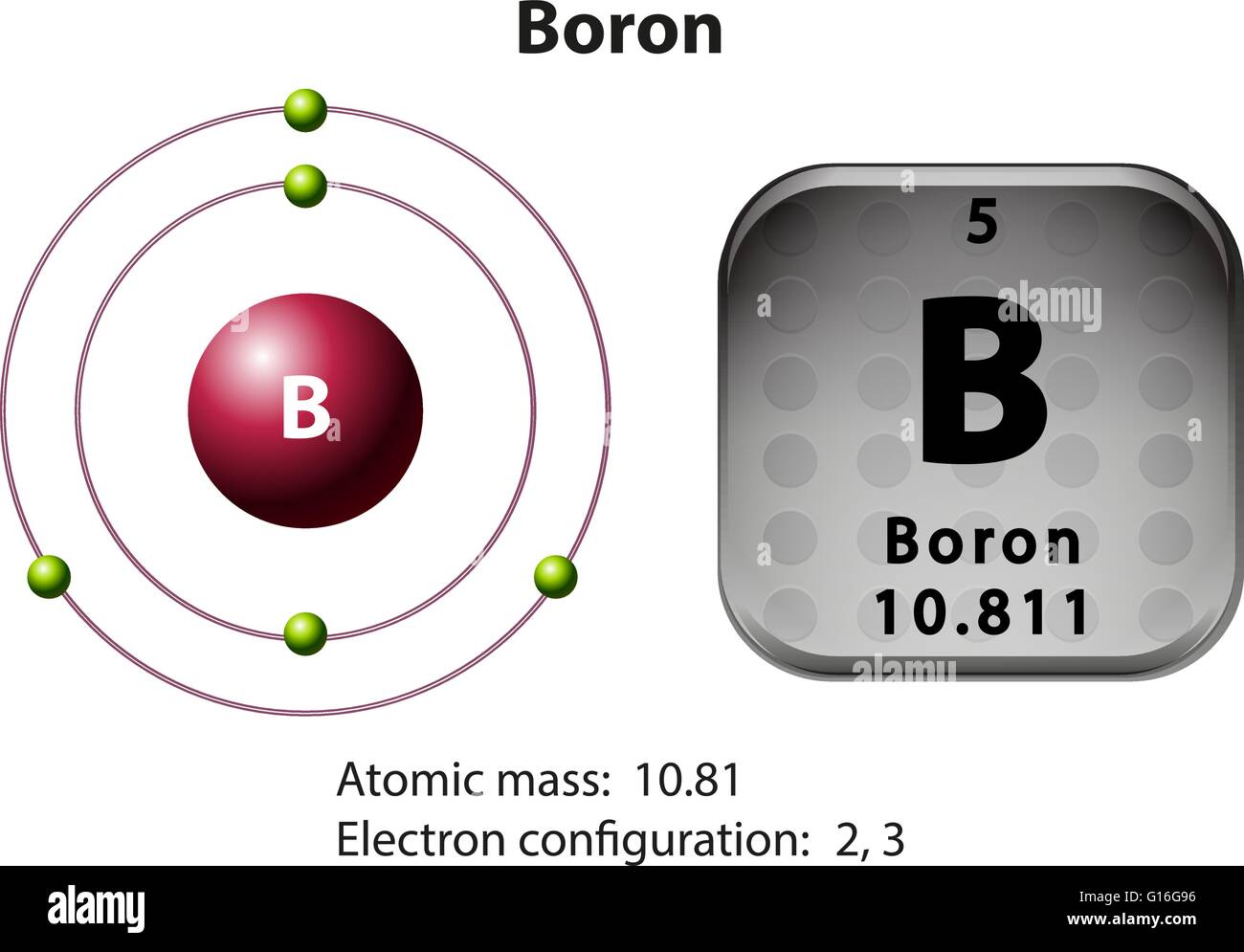
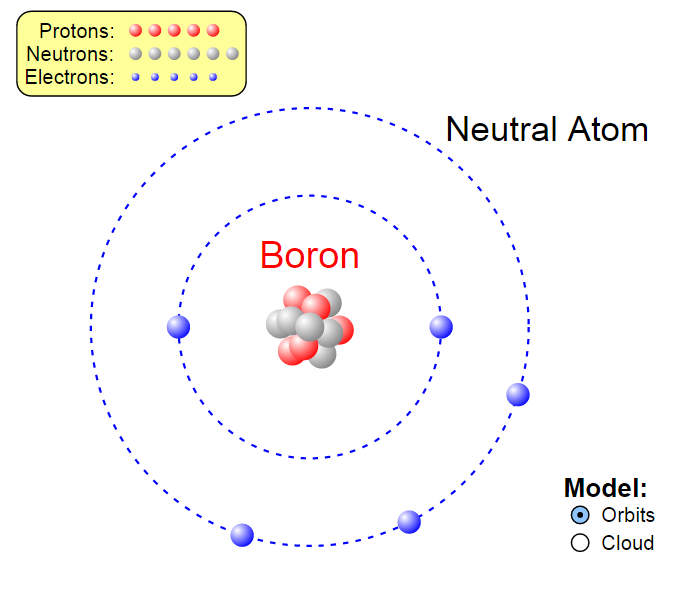
Web for each electron shell atom diagram, the element symbol is listed in the nucleus. 1s 2 2s 2 2p 3: Web the electron configuration of boron is 1s 2 2s 2 2p 1: A neutral atom has the same number of electrons as protons, so a neutral boron atom has 5 electrons. Write the.
Boron Element With Reaction, Properties, Uses, & Price Periodic Table
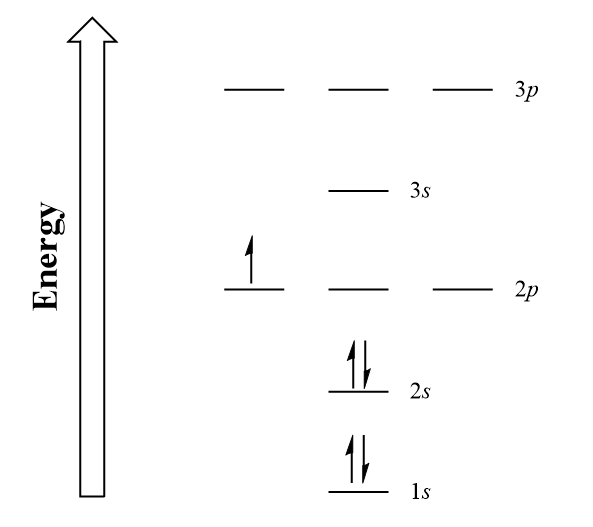
Energy 1 х 5 this problem has been solved! Energy 1 l х 5 ? 1s 2 2s 2 2p 2: Since 1s can only hold two electrons the next 2 electrons for b goes in the 2s orbital. A neutral atom has the same number of electrons as protons, so a neutral boron atom.
3d render of atom structure of boron isolated over white background
Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Beryllium has two valence electrons in its 2 shell, so its electron dot diagram is like that of helium: The charge is equal to the number of electrons that must be gained to fill. Web chemistry.
Visualizing Chemistry Activity 5
Should the sixth electron be placed in the same 2 p orbital that already has an electron, or should it go in one of the empty 2 p orbitals? Web for each electron shell atom diagram, the element symbol is listed in the nucleus. The number of the principal quantum shell, n, the letter that.
Boron Electron Configuration YouTube
Web electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. Web for each electron shell atom diagram, the element symbol is listed in the nucleus. An atom has a valence shell electron configuration of #ns^1#. 1s 2 2s 2 2p 3: The electron shells.
Boron_electron_configuration_energy_diagram Introductory Chemistry
Web draw the electron configuration for a neutral atom of boron. First ionisation energy the minimum energy required to remove an electron from a neutral atom in its. Energy 1 х 5 this problem has been solved! Electron configuration of nitrogen (n) [he] 2s 2 2p 3: Beryllium has two valence electrons in its 2.
How To Find The Boron Electron Configuration (B)
Energy х to this problem has been solved! The electron configurations and orbital diagrams of these four elements are: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web the boron atom consists of 3 electrons in its valence shell i.e. Here’s the best way to solve it..
Symbol and electron diagram Boron illustration Stock Vector Image & Art
At carbon, with z = 6 and six electrons, we are faced with a choice. Should the sixth electron be placed in the same 2 p orbital that already has an electron, or should it go in one of the empty 2 p orbitals? The element atomic number and name are listed in the upper.
Visualizing Chemistry 105 Activity 5
The electron configurations and orbital diagrams of these four elements are: 1s 2 2s 2 2p 3: Electron configuration of nitrogen (n) [he] 2s 2 2p 3: The charge is equal to the number of electrons that must be gained to fill. Since 1s can only hold two electrons the next 2 electrons for b.
Draw The Electron Configuration For A Neutral Atom Of Boron. Web boron is the fifth element with a total of 5 electrons. Electron configuration of carbon (c) [he] 2s 2 2p 2: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. Energy 1 х 5 this problem has been solved! Should the sixth electron be placed in the same 2 p orbital that already has an electron, or should it go in one of the empty 2 p orbitals?
1S 2 2S 2 2P 2:
The electron shells are shown, moving outward from the nucleus. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. An atom has a valence shell electron configuration of #ns^1#. Electron configuration through orbit (bohr principle)
Web This Electron Configuration Calculator Will Instantly Show You The Distribution Of Electrons In The Orbitals Of Any Periodic Element You Choose.
Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. This allows us to easily draw the lewis structure of boron in which the nucleus is represented using the atomic symbol of the element and the valence shell electrons are presented in the form of dots around the nucleus. Web electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. Since 1s can only hold two electrons the next 2 electrons for b goes in the 2s orbital.
Its Valence Electron Shell Is 2,.
Here’s the best way to solve it. In writing the electron configuration for boron the first two electrons will go in the 1s orbital. Web an electrically neutral atom has the following electron configuration: Web the electron configuration of boron is 1s 2 2s 2 2p 1:
A Neutral Helium Atom, With An Atomic Number Of 2 ( Z = 2), Has Two Electrons.
A neutral atom has the same number of electrons as protons, so a neutral boron atom has 5 electrons. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): 1s 2 2s 2 2p 1: