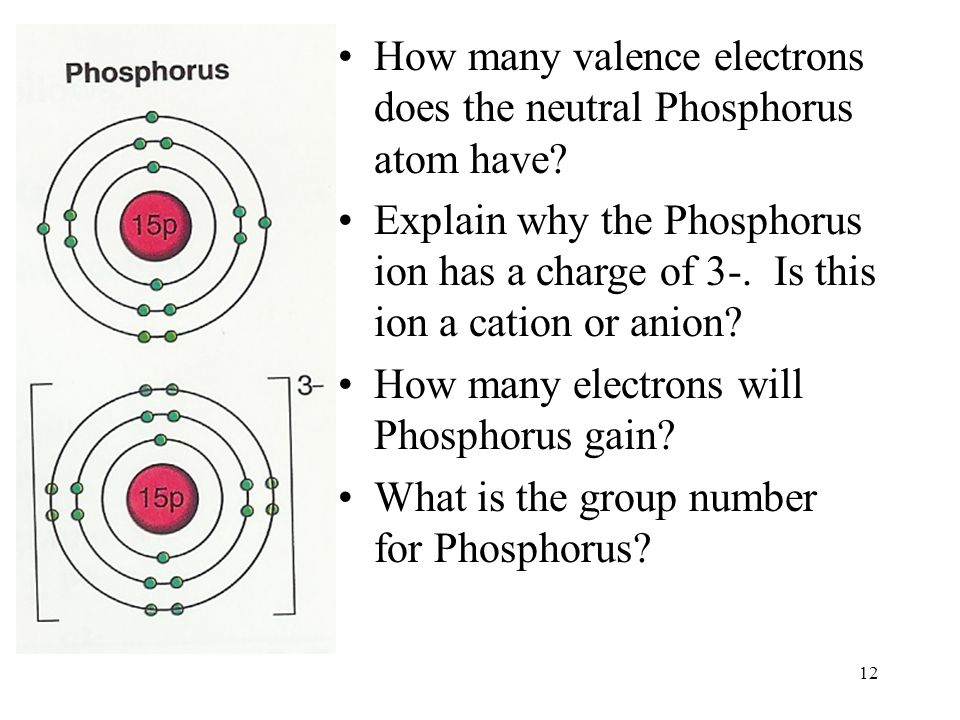
Draw The Electron Configuration For A Neutral Atom Of Phosphorus
Draw The Electron Configuration For A Neutral Atom Of Phosphorus - Write the electron configuration for a neutral atom of phosphorus. Experts have been vetted by chegg as specialists in this subject. Of those 5 electrons, 2 can go into the 3 s subshell, and the remaining 3 electrons can go into the 3 p subshell. To form bonds with the five chlorine atoms in phosphorus pentachloride, phosphorus utilizes its three 3p orbitals and two 3s orbitals. Web the final ring or shell of electrons contains the typical number of valence electrons for an atom of that element.
Electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative scale. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. Web the p orbital can hold up to six electrons. Of those 5 electrons, 2 can go into the 3 s subshell, and the remaining 3 electrons can go into the 3 p subshell. For the transition metals, electrons are removed from the s orbital first and then from the d orbital. A neutral phosphorus atom has 15 electrons. Web ignore the inner orbitals (those that correspond to the electron configuration of the nearest noble gas) and write the valence electron configuration for phosphorus.
Number Of Valence Electrons In Phosphorus
Web what is the electron configuration and orbital diagram for a phosphorus atom? Two electrons can go into the 1 s subshell, 2 can go into the 2 s subshell, and 6 can go into the 2 p subshell. Each chlorine atom contributes one electron to form a covalent bond with phosphorus. The number of.
Phosphorus Electron Configuration (P) with Orbital Diagram
Web find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. The element atomic number and name are listed in the upper left. Web march 23, 2023 by jay electron configuration chart of all elements is mentioned in the table below. Next, remove electrons from the.
Phosphorus Protons Neutrons Electrons Electron Configuration
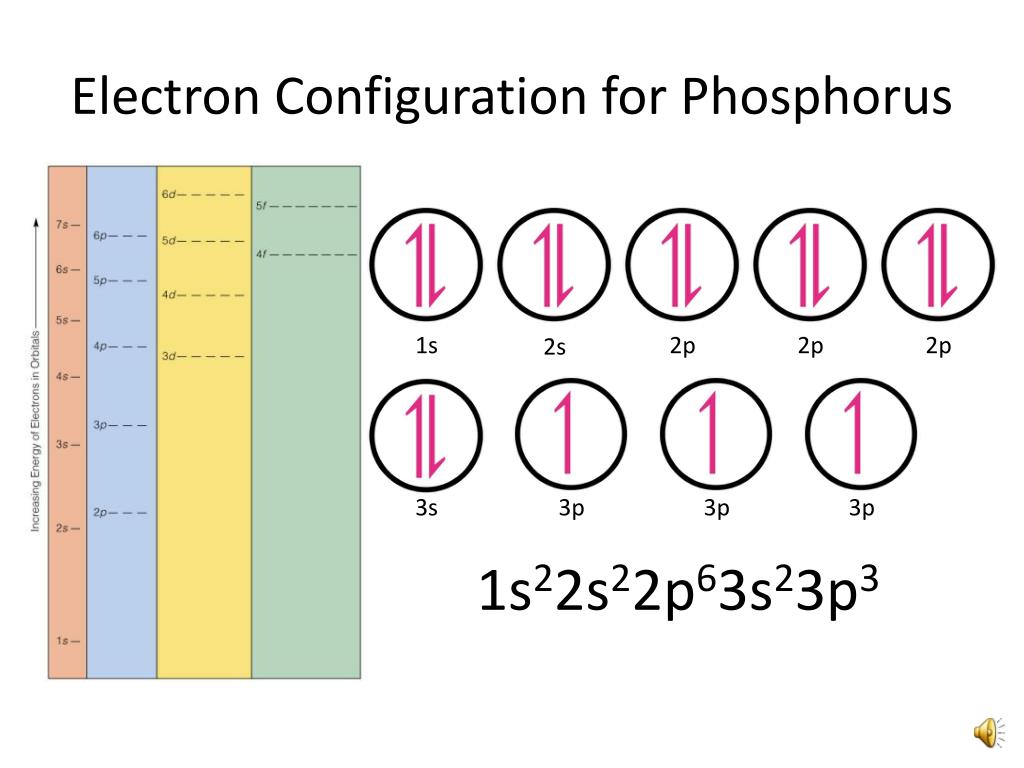
There are 2 steps to solve this one. Web the final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Thus, a phosphorus atom.
Phosphorus Electron Configuration (P) with Orbital Diagram
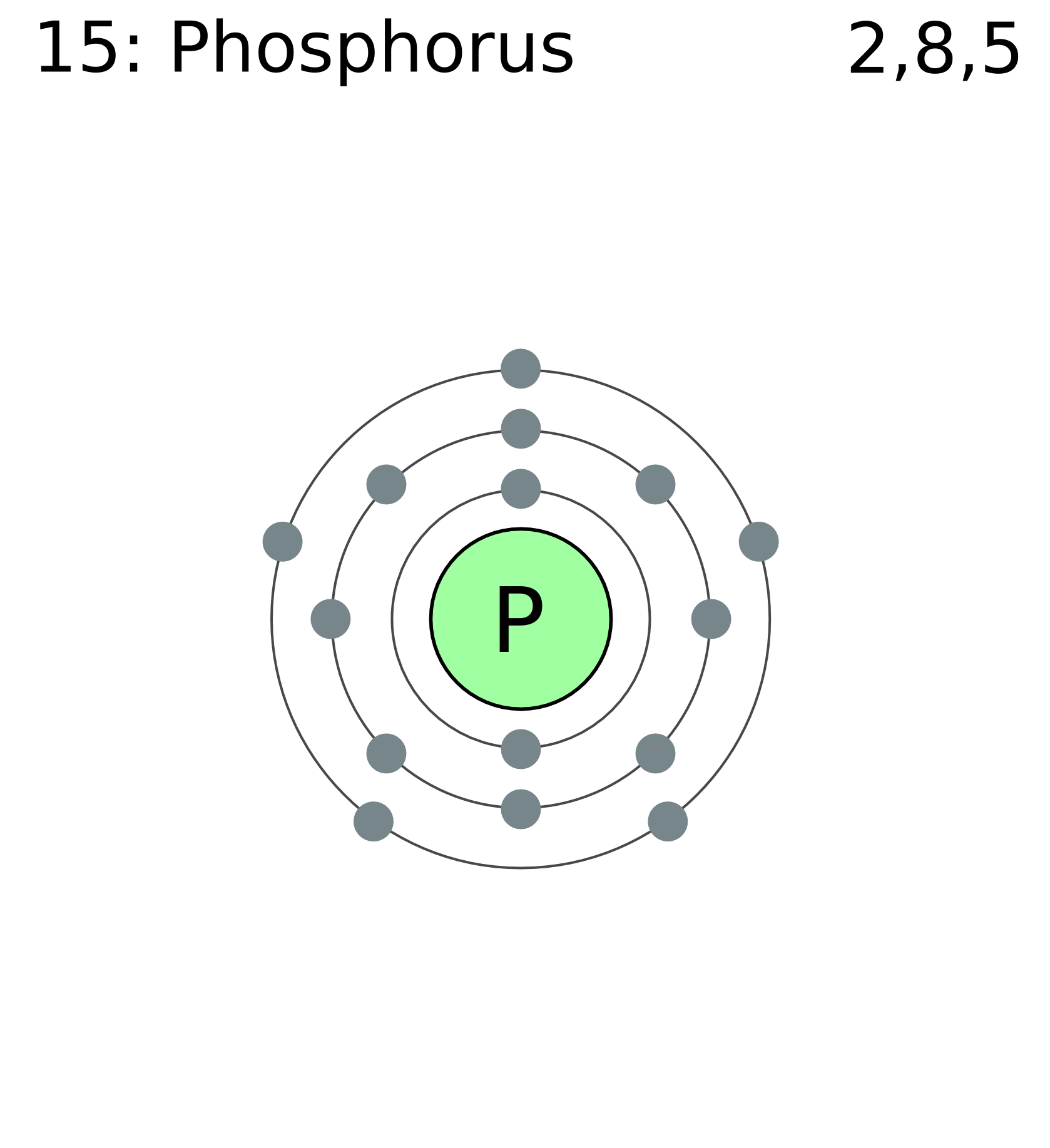
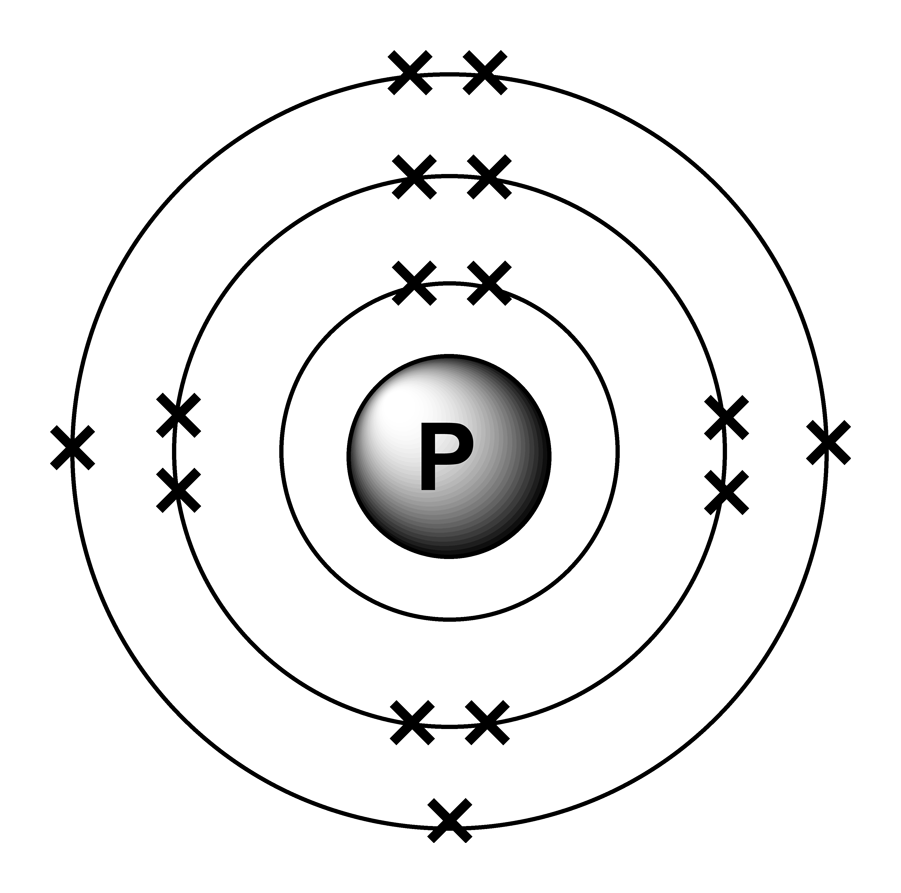
Therefore, the number of electrons in neutral atom of phosphorus is 15. Web find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Web write the electron configuration for a neutral atom of phosphorus. The element atomic number and name are listed in the upper left..
Electron configuration example for phosphorus Science, Chemistry
Web find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. This means that a neutral phosphorus atom will have 15 electrons surrounding its nucleus. Therefore, the number of electrons in neutral atom of phosphorus is 15. O electronic structure drawing a box diagram of the.
Periodic Table Phosphorus Electron Configuration Periodic Table Timeline
Web the final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Web science chemistry chemistry questions and answers write the electron configuration for a neutral atom of phosphorus. Thus, a phosphorus atom contains 15 electrons. Solution the atomic number of phosphorus is 15. To draw.
Electron arrangements
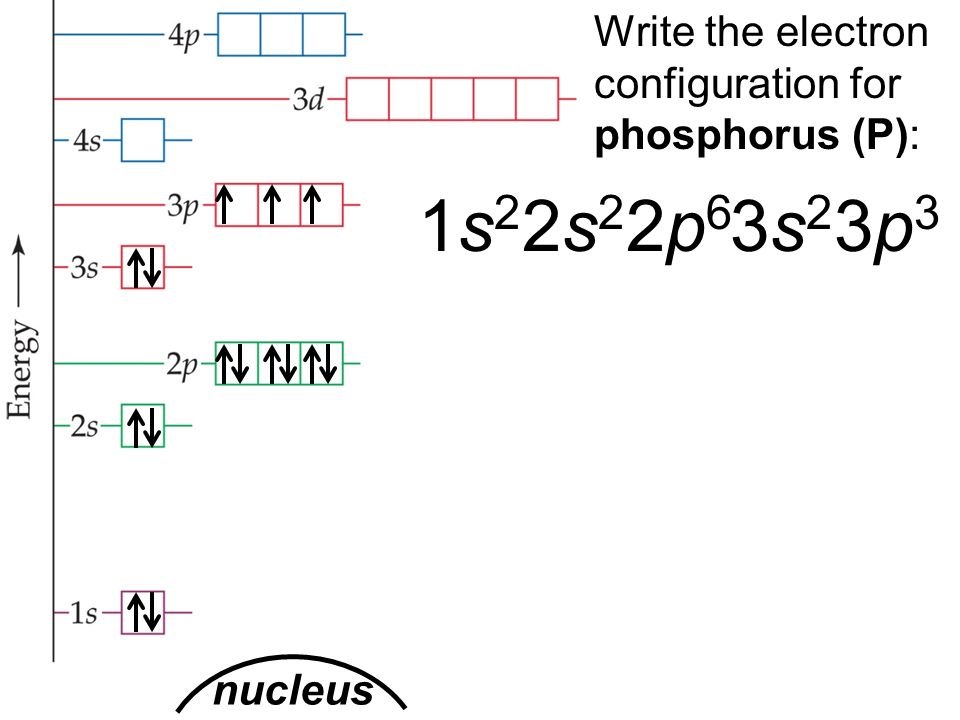
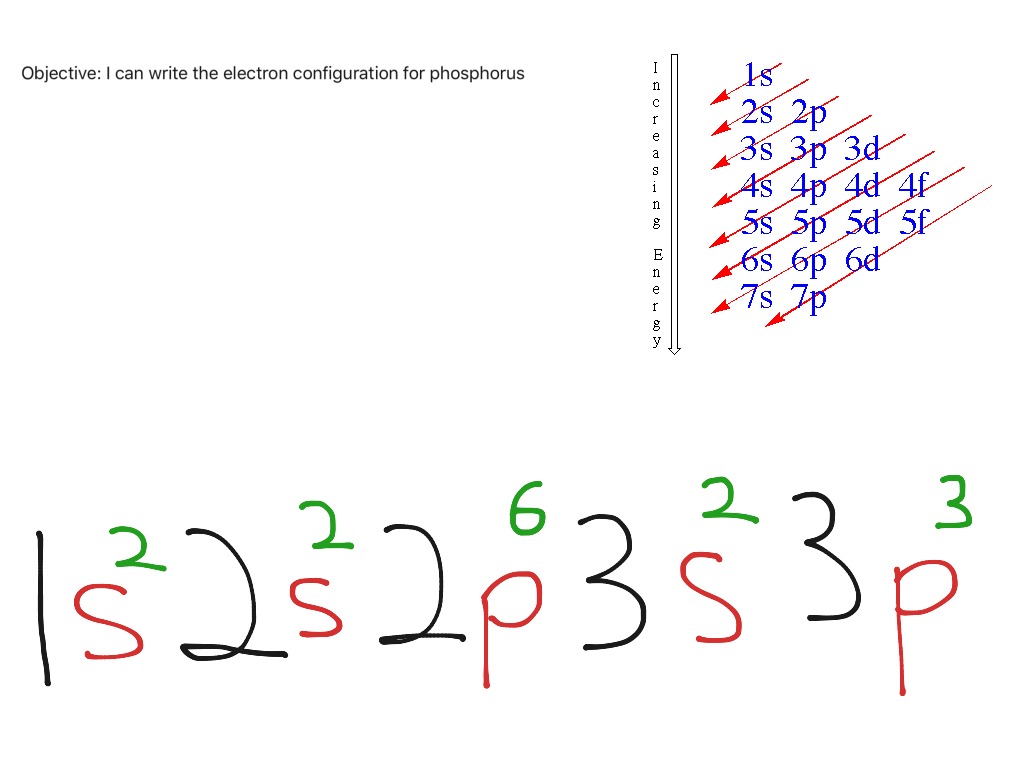
Write the electron configuration for a neutral atom of phosphorus. Phosphorus has 5 valence electrons. First ionisation energy the minimum energy required to remove an electron from a neutral atom in its. Web the electron configuration of phosphorus is [ ne] 3s 2 3p 3 , if the electron arrangement is through orbitals. Web ignore.
Atom Diagrams Electron Configurations of the Elements
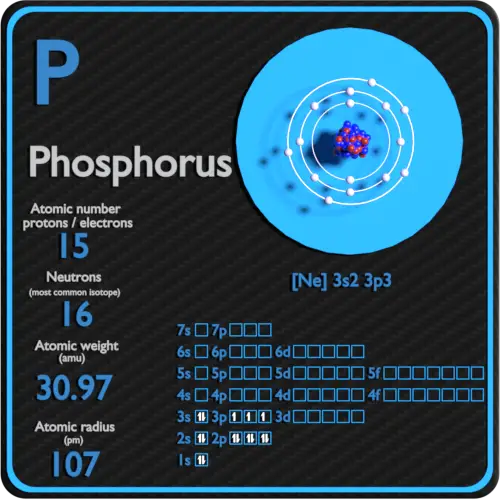
Web what is the electron configuration of a neutral phosphorus atom? Web the shorthand electron configuration for phosphorus is [ne] 3s 2 3p 3. Phosphorus is situated in group 15th or 5a and has an atomic number of 15. Therefore, the electron configuration of a neutral phosphorus atom will show 15 electrons. Write the electron.
Phosphorus Definition, Facts, Symbol, Discovery, Property, Uses
Of those 5 electrons, 2 can go into the 3 s subshell, and the remaining 3 electrons can go into the 3 p subshell. Phosphorus has 5 valence electrons. The number of valence electrons available for the phosphorus atom is 5. Two electrons can go into the 1 s subshell, 2 can go into the.
Periodic Table Phosphorus Valence Electrons Periodic Table Timeline
Remember, a neutral atom contains the same number of protons and electrons. The order of filling of the energy levels is 1s, 2s, 2p, 3s, 3p, 4s,. Write the electron configuration for a neutral atom of phosphorus. A because phosphorus is in the third row of the periodic table, we know that it has a.
Draw The Electron Configuration For A Neutral Atom Of Phosphorus Phosphorus has 5 valence electrons. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. Web science chemistry chemistry questions and answers draw the electron configuration for a neutral atom of phosphorus. Thus, a phosphorus atom contains 15 electrons. Web phosphorus, p, is located in period 3, group 15 of the periodic table, and has an atomic number equal to 15.
This Is Sometimes Called The Bohr, Or The ‘Solar System’, Model.
There are 2 steps to solve this one. Orbital diagram and valence electron configuration for phosphorus To draw lewis structures for elements, the symbol for the element is drawn with the number of valence electrons it has surrounding it. Web what is the electron configuration of a neutral phosphorus atom?
Each Chlorine Atom Contributes One Electron To Form A Covalent Bond With Phosphorus.
Solution the atomic number of phosphorus is 15. Web the final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Web draw an orbital diagram using the shorthand nobel gas configuration and use it to derive the electron configuration of phosphorus, z = 15. Web the electron configuration of phosphorus in its ground state is 1s2 2s2 2p6 3s2 3p3.
Experts Have Been Vetted By Chegg As Specialists In This Subject.
Web what is the electron configuration and orbital diagram for a phosphorus atom? Write the electron configuration for a neutral atom of phosphorus. Of those 5 electrons, 2 can go into the 3s subshell, and the remaining 3. Web find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration.
A Because Phosphorus Is In The Third Row Of The Periodic Table, We Know That It Has A [Ne] Closed Shell With 10 Electrons.
Remember, a neutral atom contains the same number of protons and electrons. Web the shorthand electron configuration for phosphorus is [ne] 3s 2 3p 3. There are 2 steps to solve this one. The element atomic number and name are listed in the upper left.


:max_bytes(150000):strip_icc()/phosphorusatom-58b6025c5f9b5860464c65dc.jpg)