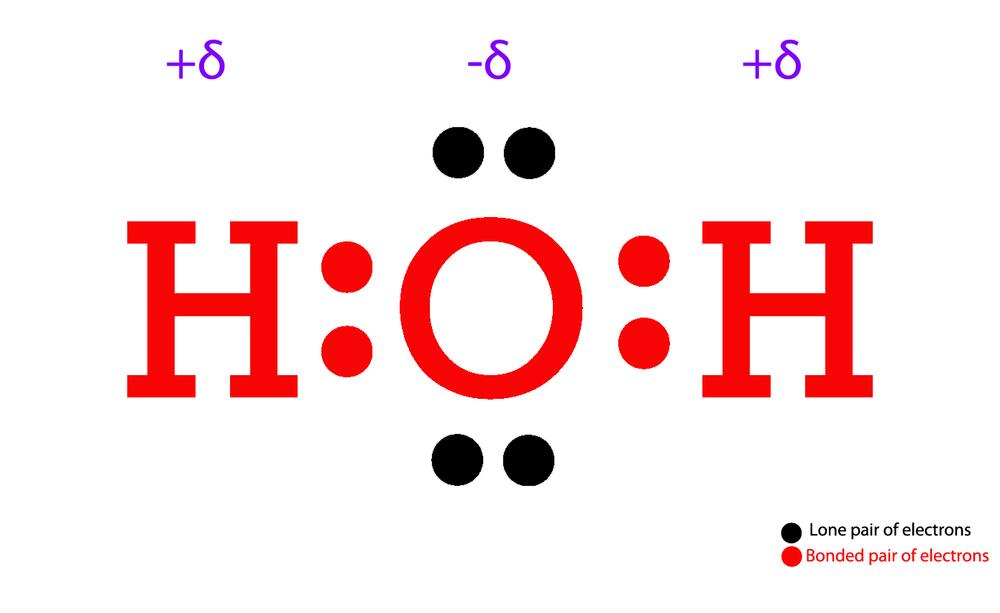
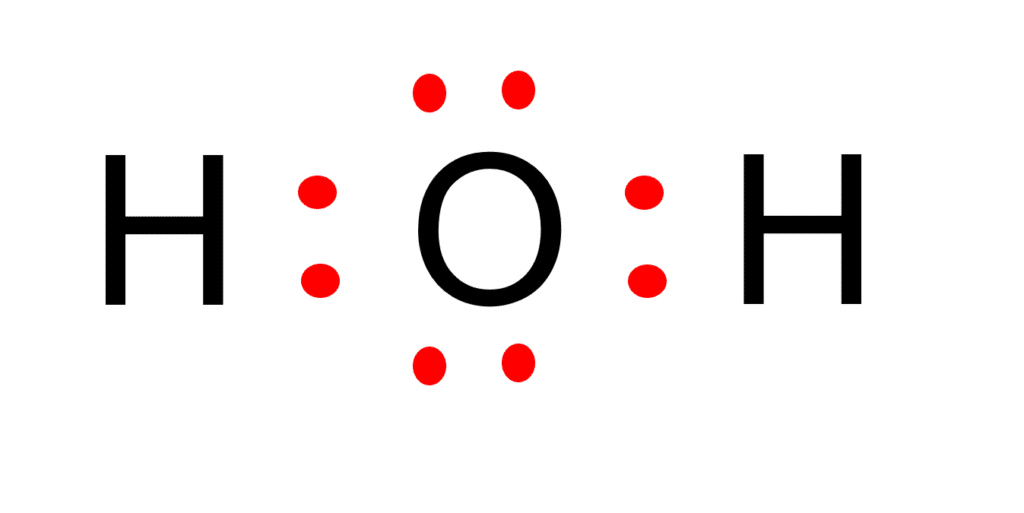
Draw The Lewis Dot Structure For H2O
Draw The Lewis Dot Structure For H2O - First, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of. The lewis structure helps us understand the bonding and electron distribution in water, which is essential for understanding its chemical properties. For the h2o structure use the periodic table to find the total number of valence electrons for the. The steps that must be followed while drawing a lewis structure are listed below. Web some structures don't obey the octet rule, but explain why.
Drawing the lewis dot structure for h 2 o and answer the questions below. I also go over hybridization, shape and bond angle. Web what are lone pairs and how are they represented in a lewis dot diagram? The sum of the valence electrons is. Web the molecular geometry of any molecule depends on its lewis structure, the arrangement of atoms, and its electrons. Web how to draw lewis structure of h2o. First, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of.
H2O Lewis structure and Molecular Geometry [No1 Best Explanation
Web watch the video of dr. Is it possible to draw lewis dot diagrams for ionic compounds? Lewis structure of h2 (or diatomic hydrogen) contains a single bond between both the hydrogen (h) atoms. Also, there are two lone pairs on oxygen atom. Web how to draw lewis structure for h2o 1. The h 2.
H2O Lewis structure and Molecular Geometry [No1 Best Explanation
We have a total of eight valence electrons. Lewis structure of h2 (or diatomic hydrogen) contains a single bond between both the hydrogen (h) atoms. Is it possible to draw lewis dot diagrams for ionic compounds? Web 6 steps to draw the lewis structure of h2o step #1: While selecting the center atom, always put.
H2O Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Lewis structure of h2 (or diatomic hydrogen) contains a single bond between both the hydrogen (h) atoms. A common error it to put two oxygen atoms and one hydrogen making ho 2. The steps that must be followed while drawing a lewis structure are listed below. For the h2o structure use the periodic table to.
Water Lewis Structure How to Draw the Lewis Structure for Water YouTube
In order to draw the. A video explanation of how to draw the lewis dot structure for water, along with information about the compound including formal charges, polarity, hybrid orbitals, shape,. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. Determine the total number of valence (outer shell) electrons..
Lewis Dot Diagram For H2o Free Diagram For Student
The sum of the valence electrons is. For the h2o structure use the periodic table to find the total number of valence. For the h2o structure use the periodic table to find the total number of valence electrons for the. Water (h2o) is a molecule composed of two hydrogen atoms bonded to a central oxygen.
H2O Lewis Structure, Molecular Geometry, and Hybridization
Let’s draw and understand this lewis dot structure in simple steps. On the periodic table, hydrogen's in group 1, it has 1 valence electron; Web 33k views 3 days ago. Hydrogen is a group ia. Here, the given molecule is h2o (water). For the h2o structure use the periodic table to find the total number.
In this video we are going to learn about the Lewis structure of H2O
The h 2 o lewis dot structure is seen fairly frequently. Web how to draw lewis structure for h2o 1. Web 33k views 3 days ago. Web let's do the lewis structure for water: Draw lewis dot structures for ch4 ch 4, nh3 nh 3, hf hf, of2 of 2, f2 f 2, o2 o.
Draw Step By Step The Lewis Structure For Water (H2O)
So, if you are ready to go with these 6 simple steps, then let’s dive right into it! Water molecule is a simple molecule. Lewis structure of h2o2 (or hydrogen peroxide) contains single bonds between the two oxygen (o) atoms as well as between oxygen (o) & hydrogen (h) atoms. The sum of the valence.
Lewis Structures Hydrogen (H2), and Water (H2O) What's Insight
Water (h2o) is a molecule composed of two hydrogen atoms bonded to a central oxygen atom. Is it possible to draw lewis dot diagrams for ionic compounds? Calculate the total number of valence electrons. Here, the given molecule is h2o (water). The steps that must be followed while drawing a lewis structure are listed below..
H2O Lewis Structure, Molecular Geometry, and Hybridization
Steps to draw the lewis structure of h2o. The lewis structure helps us understand the bonding and electron distribution in water, which is essential for understanding its chemical properties. Select the center atom (h is always outside). Hydrogen is a group ia. Web the molecular geometry of any molecule depends on its lewis structure, the.
Draw The Lewis Dot Structure For H2O O has 6 valence electrons, and each h has one. The h 2 o lewis dot structure is seen fairly frequently. Water molecule is a simple molecule. Draw lewis dot structures for ch4 ch 4, nh3 nh 3, hf hf, of2 of 2, f2 f 2, o2 o 2, n2 n. Web the molecular geometry of any molecule depends on its lewis structure, the arrangement of atoms, and its electrons.
Determine The Total Electron Pairs In The Form Of Lone Pairs And Bonds.
Also, there are two lone pairs on oxygen atom. And oxygen is in group 6, sometimes called 16, so it has 6 valence electrons. Calculate the total number of valence electrons. The lewis structure helps us understand the bonding and electron distribution in water, which is essential for understanding its chemical properties.
Web The Molecular Geometry Of Any Molecule Depends On Its Lewis Structure, The Arrangement Of Atoms, And Its Electrons.
Determine the total number of valence (outer shell) electrons. Web each step of drawing lewis structure of h 2 o are explained in this tutorial. But we have two of them, so let's multiply that by 2. Select the center atom (h is always outside).
In Order To Draw The.
Assign formal charges to atoms in the structure. Hydrogen atoms are joint to oxygen atom through single bonds. So 1 times 2 is 2, plus 6; Lewis structure of h2o2 (or hydrogen peroxide) contains single bonds between the two oxygen (o) atoms as well as between oxygen (o) & hydrogen (h) atoms.
A Video Explanation Of How To Draw The Lewis Dot Structure For Water, Along With Information About The Compound Including Formal Charges, Polarity, Hybrid Orbitals, Shape,.
We have a total of eight valence electrons. Let’s draw and understand this lewis dot structure in simple steps. First, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of. What is the lewis dot diagram for #h_2o#?