Draw The Lewis Structure For A Carbon Monosulfide Cs Molecule
Draw The Lewis Structure For A Carbon Monosulfide Cs Molecule - Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. ° = a + b*t + c*t h° − h° = a*t + b*t /2 + c*t /3 + d*t /4 − e/t + f − h s° = a*ln (t) + b*t + c*t /2 + d*t /3 − e/ (2*t c = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy (j/mol*k) t. Web draw lewis structures depicting the bonding in simple molecules lewis structures Web the lewis structure for a carbon monosulfide (cs) molecule and ar are attached. Counting total valence electrons of atoms.
The o has two bonding pairs and two lone pairs, and c has four bonding pairs. Web the carbon monosulfide lewis structure consists of a carbon atom bonded to a sulfur atom. Dinah zike, laurel dingrando, nicholas hainen, cheryl wistrom publisher: So we have eight remaining. Lewis structures are also called as electron dot structures and can be drawn if the molecular formula of a compound is known. It provides information regarding the nature of bond and the position of atoms. Constructing lewis electron structures 1.
Lewis Structure Types
The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. The oxygen also has two lone pairs drawn. Constructing lewis electron structures 1. Web the following procedure can be used to construct lewis electron structures for simple molecules. This diatomic molecule is the sulfur.
Carbon Monosulfide Lewis Dot Structure bmptootles
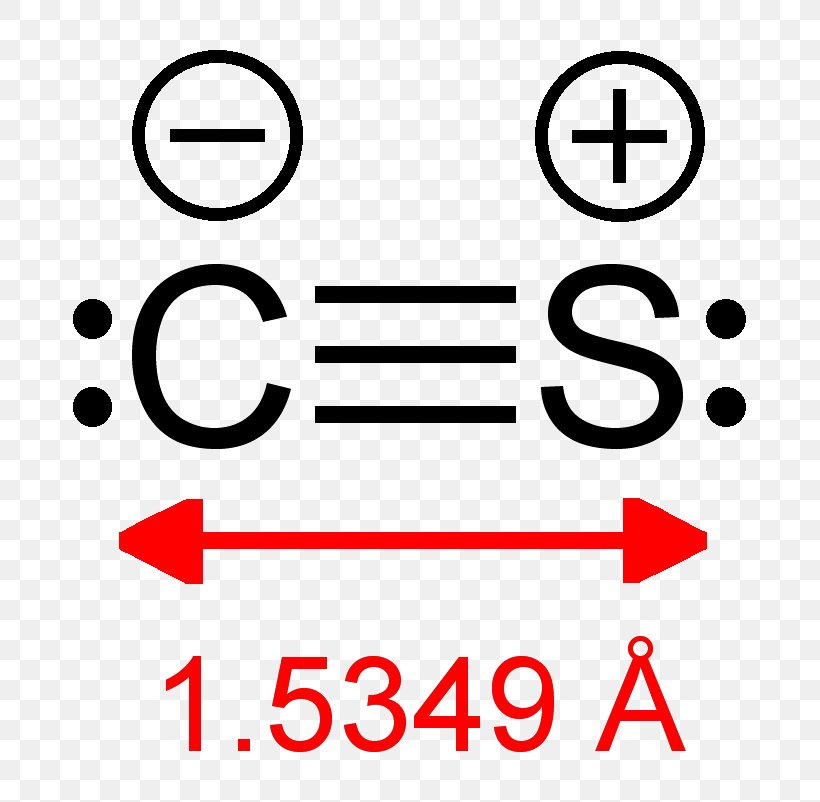
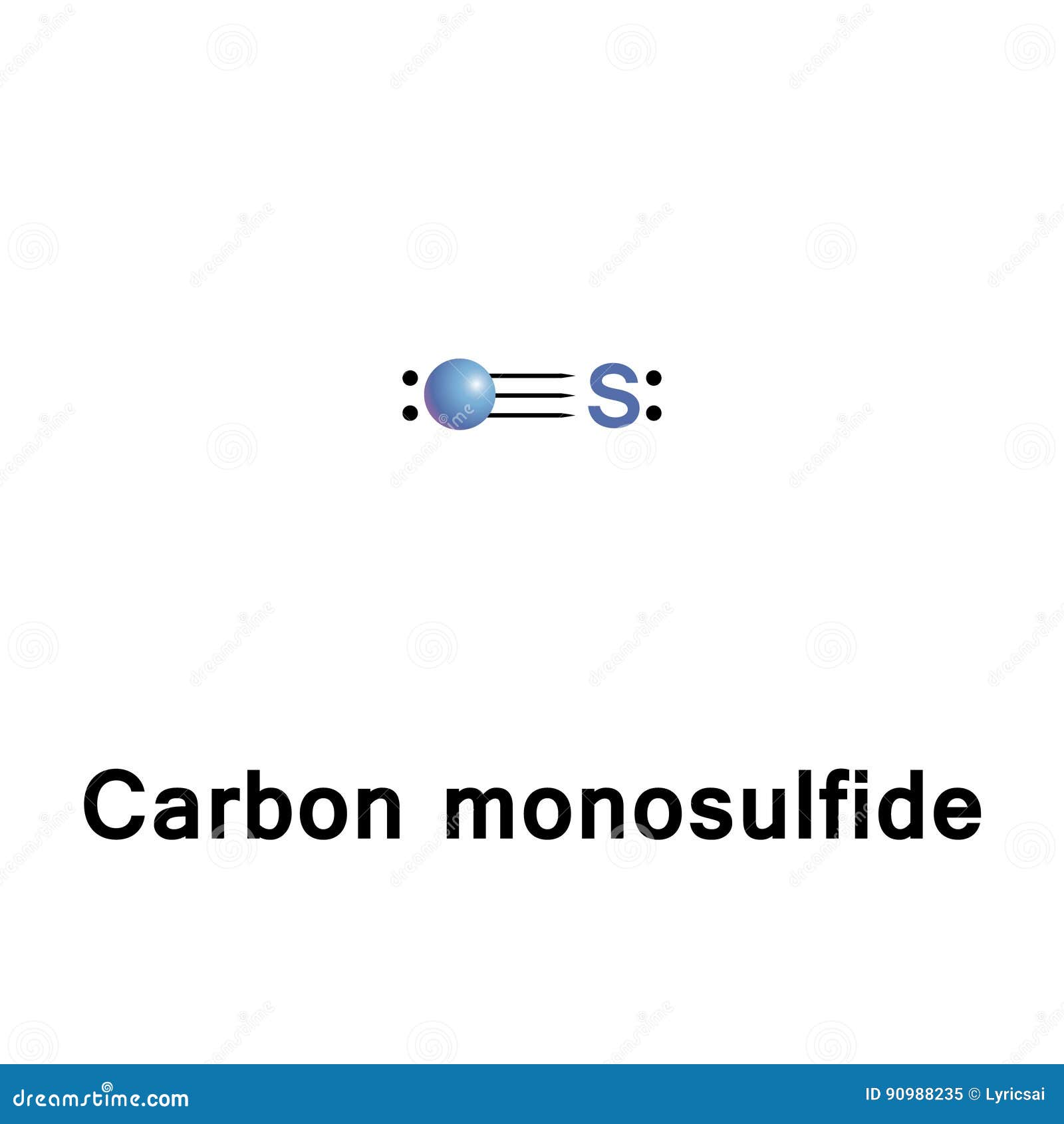
Draw the lewis structure for a carbon monosulfide molecule. Therefore, there are a total of ten valence electrons present in the cs molecule (six plus four equals ten). To complete their octets, the carbon atom shares two electrons with the sulfur atom, forming a double bond. Deperturbed constants barrow, dixon, et al., 1961; Web draw.
Lewis Diagram Of Carbon
Web science chemistry draw the lewis structure for a carbon monosulfide (cs) molecule. Web the bond between the oxygen and carbon is replaced with a double bond. Web verified answer carbon monosulfide lewis structure So we have eight remaining. Constructing lewis electron structures 1. Web writing lewis structures for diatomic molecules draw the lewis structure.
[Solved] Choose the Lewis dot formula that most accurately describes
Web drawing lewis structures for molecules with one central atom: Then what we do is we bond the carbon to the sulfur. Web draw the lewis dot diagram for carbon. The oxygen also has two lone pairs drawn. Matter and change 1st edition isbn: Therefore, there are a total of ten valence electrons present in.
Carbon Monosulfide Lewis Structure Molecule Carbon Monoxide, PNG
Web draw lewis structures depicting the bonding in simple molecules lewis structures It consists of one carbon and one sulphur atom. Deperturbed constants barrow, dixon, et al., 1961; So we have eight remaining. Co x 5 this problem has been solved! Draw lewis structures depicting the bonding in simple molecules. Before we can begin drawing.
CS2 Lewis Structure, Hybridization, Polarity and Molecular Shape
Web science chemistry draw the lewis structure for a carbon monosulfide (cs) molecule. Web the following procedure can be used to construct lewis electron structures for simple molecules. To complete their octets, the carbon atom shares two electrons with the sulfur atom, forming a double bond. To count the valence electrons in cos,. Constructing lewis.
How to Draw Lewis Structures
That gives us a total of ten valence electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw lewis structures depicting the bonding in simple molecules. Web draw the lewis structure for a carbon monosulfide (cs) molecule 谲 alo ar this problem has been solved! Web write.
Carbon Monosulfide Lewis Dot Structure bmptootles
[1] the molecule resembles carbon monoxide with a triple bond between carbon and sulfur. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Carbon has four and sulfur has six. Web writing lewis structures for diatomic molecules draw the lewis.
FileCarbon Lewis Structure PNG.png Wikimedia Commons
Dinah zike, laurel dingrando, nicholas hainen, cheryl wistrom publisher: Draw the lewis structure for a carbon monosulfide (cs) molecule. Before we can begin drawing the lewis structure for cos (carbon monosulfide), we need to determine the total number of valence electrons present in the molecule.valence electrons are the outermost electrons of an atom that participate.
Carbon Monosulfide Molecule Stock Vector Illustration of sphere
It is an organo sulphur compound. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw lewis structures for covalent compounds. Web draw lewis structures depicting the bonding in simple molecules lewis structures Draw the lewis structure for a carbon monosulfide molecule. Web write lewis symbols for neutral.
Draw The Lewis Structure For A Carbon Monosulfide Cs Molecule The o has two bonding pairs and two lone pairs, and c has four bonding pairs. That gives us a total of ten valence electrons. Dinah zike, laurel dingrando, nicholas hainen, cheryl wistrom publisher: An atom of carbon has a total of 4 valence electrons, while an atom of sulfur has a total of 6 valence electrons. Both the oxygen and the carbon now have an octet of electrons, so this is an acceptable lewis electron structure.
Lewis Structures Are Also Called As Electron Dot Structures And Can Be Drawn If The Molecular Formula Of A Compound Is Known.
It provides information regarding the nature of bond and the position of atoms. Draw the lewis structure for a carbon monosulfide molecule. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Both the oxygen and the carbon now have an octet of electrons, so this is an acceptable lewis electron structure.
Figure Out How Many Electrons The Molecule Must Have, Based On The Number Of Valence Electrons In Each.
Data last reviewed in december, 1976. The carbon atom has two valence electrons, while the sulfur atom has six valence electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. In doing so, that bond uses up two valence electrons.
Draw Lewis Structures For Covalent Compounds.
Draw lewis structures depicting the bonding in simple molecules. This diatomic molecule is the sulfur analogue of carbon monoxide, and is unstable as a solid or a liquid, but it has been observed as a gas both in the laboratory and in the interstellar medium. Counting total valence electrons of atoms. Web the carbon monosulfide lewis structure consists of a carbon atom bonded to a sulfur atom.
Draw And Explain The Lewis Dot Structure Of Carbon.
To complete their octets, the carbon atom shares two electrons with the sulfur atom, forming a double bond. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Web draw the lewis structure for a carbon monosulfide (cs) molecule 谲 alo ar this problem has been solved! Co x 5 this problem has been solved!