Draw The Lewis Structure For A Nitrogen N2 Molecule
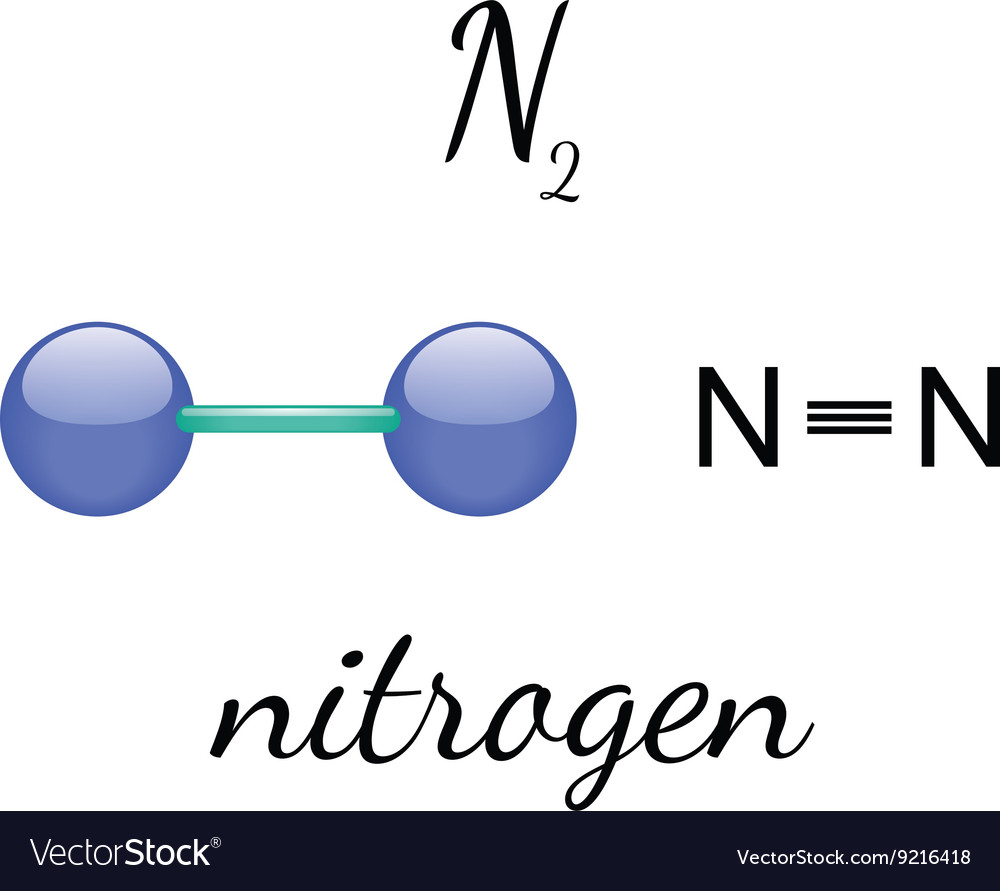
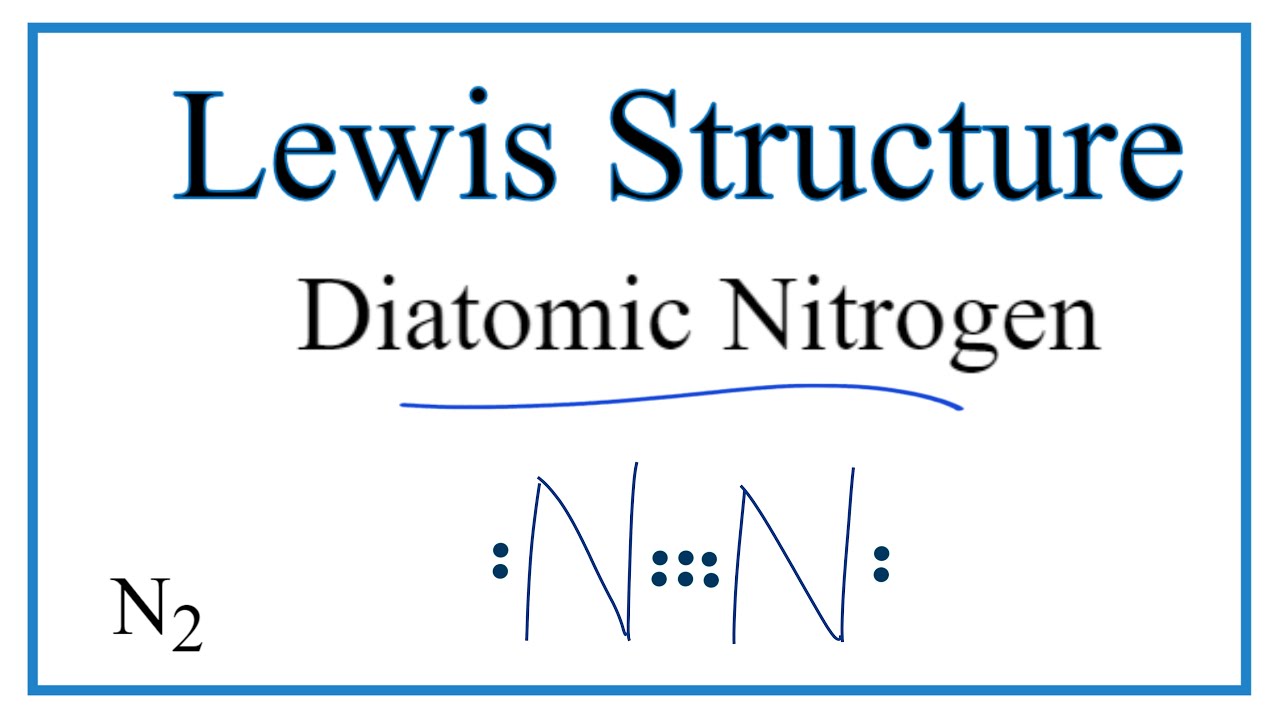
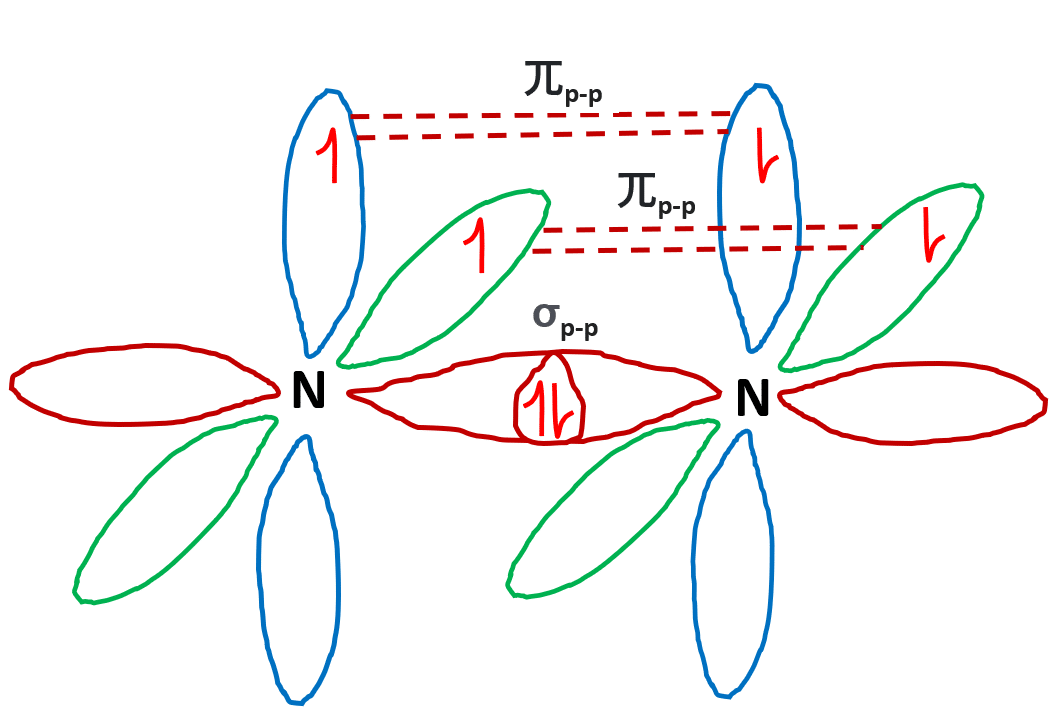
Draw The Lewis Structure For A Nitrogen N2 Molecule - Web in the lewis structure of n2, there is a triple bond between two nitrogen atoms. Web chemistry chemistry questions and answers draw the lewis structure for a nitrogen molecule, n2. Web drawing the lewis structure for n 2 (dinitogen or nitrogen gas) nitrogen (n 2 ) is a commonly tested lewis structure due to its importance on earth (about 78% of the earth's atomsphere is n 2 ). The leftover two 2p orbitals become two π bonds and electrons making a pair between the nitrogen atoms will make a sigma bond. For the n2 structure use the periodic table to find the total number of valence.
This problem has been solved! Web chemistry chemistry questions and answers draw the lewis structure for a nitrogen molecule, n2. In order to draw the lewis structure of n2, first of all you have to find the total number of valence electrons present in the n2 molecule. The n2 lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the n2 molecule. Web watch on steps of drawing n2 lewis structure step 1: There are many things to learn when we draw n2 lewis structure. Web the total number of valence electrons between the two n atoms is 10e−.
Lewis structure of N2 (Nitrogen gas) YouTube
Find the total valence electrons for the n2 molecule. The atomic number of nitrogen is 7 having an electronic configuration of 1s 2, 2s 2, 2p 3. The nuclei contain the protons and neutrons, which are the solid parts of the molecule. N2 is a colorless, odorless, and tasteless gas. Here, the given molecule is.
Lewis Dot Structure For N2 Draw Easy
Nitrogen is a diatomic molecule and contains only two nitrogen atoms. To draw the lewis structure of n2, first to find out the valance electron of nitrogen. (0) here we draw the n2 lewis structure, molecular geometry, and hybridization of nitrogen molecule. Web hey everyone, welcome to the mentor center! There are many things to.
N2 nitrogen molecule Royalty Free Vector Image
Web watch on steps of drawing n2 lewis structure step 1: In order to come up with this answer, you first need to know the number of valence electrons for nitrogen. Follow along using the transcript. Next, we need to connect the atoms in the correct order and add the electrons as bonds and lone.
N2 Lewis Structure How to Draw the Lewis Structure for N2 Nitrogen Gas
N2 is a colorless, odorless, and tasteless gas. The leftover two 2p orbitals become two π bonds and electrons making a pair between the nitrogen atoms will make a sigma bond. In order to draw the lewis structure of n2, first of all you have to find the total number of valence electrons present in.
How to Draw the Lewis Dot Structure for Diatomic Nitrogen (N2) YouTube
When the substance of nitrogen melts, what interaction (s) are overcome? Lewis structures are a way to represent the arrangement of atoms and electrons in a molecule or ion. Each nitrogen atom is surrounded by a lone pair of electrons. Follow along using the transcript. It also is a good example of a molecule with.
What is the Lewis structure of N2? Socratic
Each nitrogen atom is surrounded by a lone pair of electrons. (0) here we draw the n2 lewis structure, molecular geometry, and hybridization of nitrogen molecule. Calculate the total number of valence electrons. The atomic number of nitrogen is 7 having an electronic configuration of 1s 2, 2s 2, 2p 3. In order to draw.
Lewis Electron Dot Diagram Of N2 slide share
The leftover two 2p orbitals become two π bonds and electrons making a pair between the nitrogen atoms will make a sigma bond. Web drawing lewis structures for molecules with one central atom: Here, the given molecule is n2 (nitrogen gas). (0) here we draw the n2 lewis structure, molecular geometry, and hybridization of nitrogen.
Lewis Structure for N2 How To Discuss
Web draw the lewis structure for a nitrogen (n2) molecule. Web the first step is to sketch the lewis structure of the n2 molecule, to add valence electrons around the two nitrogen atoms, and the final step is to combine the two nitrogen diatomic atoms to get the n2 lewis structure. Nitrogen is a diatomic.
N2 Lewis Structure Hybridization & Molecular Geometry What's Insight
Web watch on steps of drawing n2 lewis structure step 1: The leftover two 2p orbitals become two π bonds and electrons making a pair between the nitrogen atoms will make a sigma bond. In order to draw the lewis structure of n2, first of all you have to find the total number of valence.
Nitrogen, N2 molecule model and chemical formula. Also dinitrogen
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web so, nitrogen is in group 5a, and therefore, it has 5 valence electrons, thus n 2 has 10 valence electrons. In order to come up with this answer, you first need to know the number of valence electrons.
Draw The Lewis Structure For A Nitrogen N2 Molecule You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The nuclei contain the protons and neutrons, which are the solid parts of the molecule. For the n2 structure use the periodic table to find the total number of valence. It also is a good example of a molecule with a triple bond.
Calculate The Total Number Of Valence Electrons.
Web so, nitrogen is in group 5a, and therefore, it has 5 valence electrons, thus n 2 has 10 valence electrons. This problem has been solved! Web there is a triple bond in lewis structure of n 2 molecule. Web drawing lewis structures for molecules with one central atom:
Web Draw The Lewis Structure For A Nitrogen (N2) Molecule.
The molecular geometry of n2 is linear. In the case of n2, each nitrogen atom has five valence electrons, so the total number of valence electrons is 10. Here, the given molecule is n2 (nitrogen gas). Determine the total number of valence electrons in the molecule by adding the valence electrons of each nitrogen atom.
The Number Of Electrons In The Valence Shell Of A Nitrogen Atom Is 5.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web hey everyone, welcome to the mentor center! In today's video, i draw the lewis dot structure of nitrogen gas (n2) and determine whether it is polar or nonpol. In order to draw the lewis structure of n2, first of all you have to find the total number of valence electrons present in the n2 molecule.
The N2 Lewis Structure Is A Diagram That Illustrates The Number Of Valence Electrons And Bond Electron Pairs In The N2 Molecule.
N2 is a colorless, odorless, and tasteless gas. Write the correct skeletal structure for the molecule. Also, for the structure to be correct, it must follow the octet rule (eight electrons per atom). Web in the lewis structure of n2, there is a triple bond between two nitrogen atoms.