Draw The Lewis Structure For Bro3-
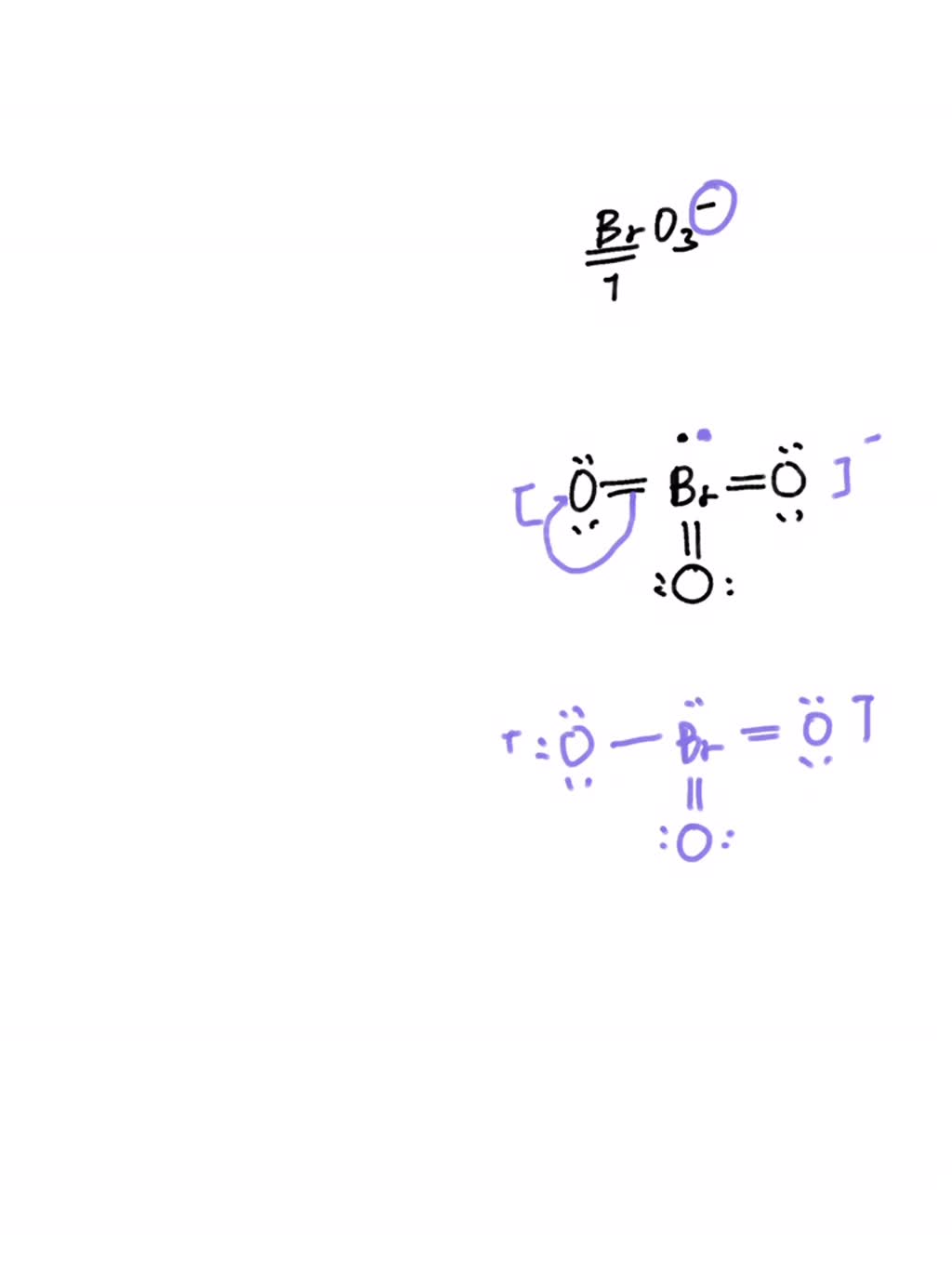
Draw The Lewis Structure For Bro3- - 7 + 3 (6) + 1= 26 valence electrons. Draw the lewis structure for bro3 check all that apply. Web this problem has been solved! Web drawing lewis structures for molecules with one central atom: Drawing lewis structures for bf3, pf3 and brf3;
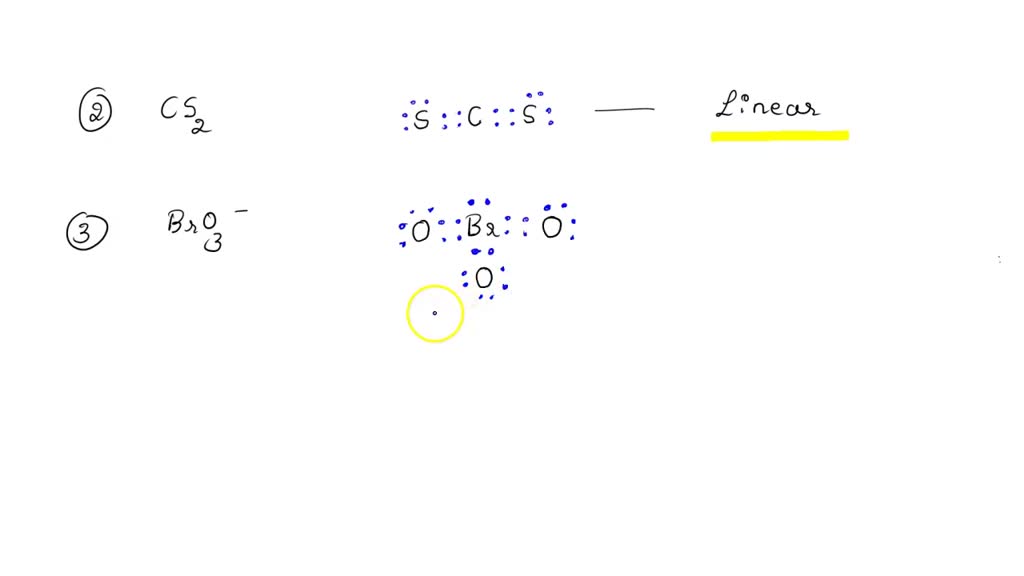
Web a video explanation of how to draw the lewis dot structure for the bromate ion, along with information about the compound including formal charges, polarity, hybrid orbitals, shape, and bond. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Adding them up, we get a total of 26 valence electrons. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms #4 minimize formal charges by converting lone pairs of the atoms, and try to get a stable lewis structure #5 repeat step 4 again if needed,. While selecting the atom, always put the least electronegative atom at the center. Web a) draw all the possible lewis structures for the following ions: And this is an anion that carries one negative charge.
Bro3 Lewis Structure
Breaking the octet rule ; Adding them up, we get a total of 26 valence electrons. Web chemistry chemistry questions and answers draw the lewis structure for the bromate ion (bro3−)with minimized formal charges. While selecting the atom, always put the least electronegative atom at the center. For the hbro3 structure use the periodic table.
BrO3 lewis structure, molecular geometry, bond angle, polarity, electrons
Adding them up, we get a total of 26 valence electrons. Calculate the total number of valence electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Breaking the octet rule ; Web drawing lewis structures for molecules with one central atom: Indicate the total number of valence.
Bro3 Lewis Structure
Web drawing lewis structures for molecules with one central atom: Valence electrons are the outermost electrons of an atom and are involved in bonding. Breaking the octet rule ; Calculate the total number of valence electrons. There are seven valence electrons in each bromine atom and six valance electrons in each oxygen atom. You'll get.
BrO3 (Bromate ion) Molecular Geometry, Bond Angles YouTube
D) indicate the approximate bond angles for each ion e) determine the names for the molecular geometry. Web drawing lewis structures for molecules with one central atom: At first we have to calculate the valence electrons in bromate ion. For the hbro3 structure use the periodic table to find the total number of valence electrons.
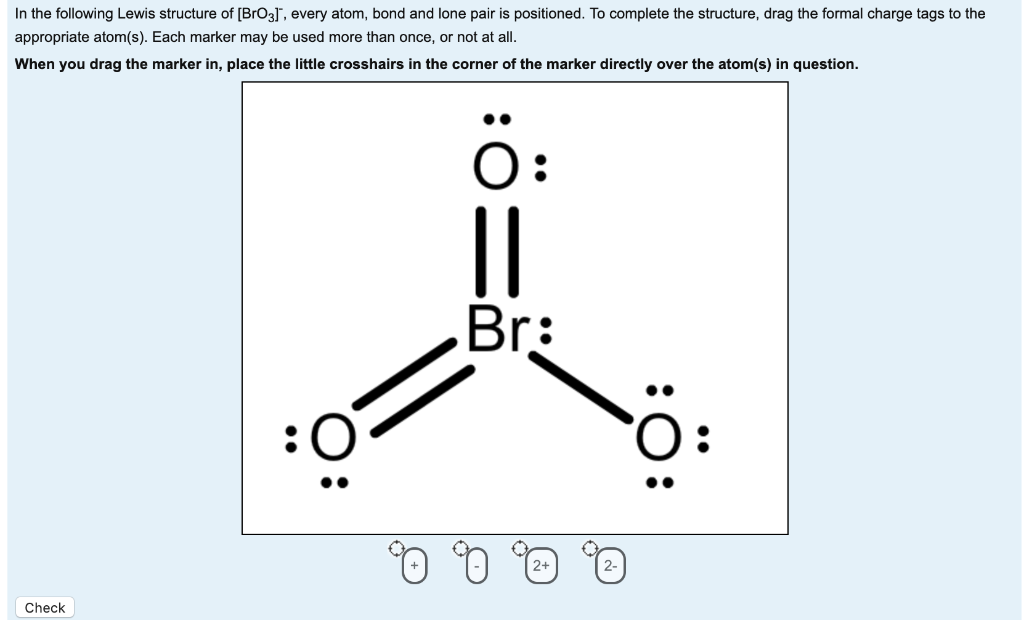
[Solved] Draw resonance Lewis structures for bromate(V), [BrO3] −
Breaking the octet rule ; First, we determine the total number of valence electrons in the molecule. 7 in a lewis structure for a neutral molecule, the. Adding them up, we get a total of 26 valence electrons. Give the name of the ion. Web a) draw all the possible lewis structures for the following.
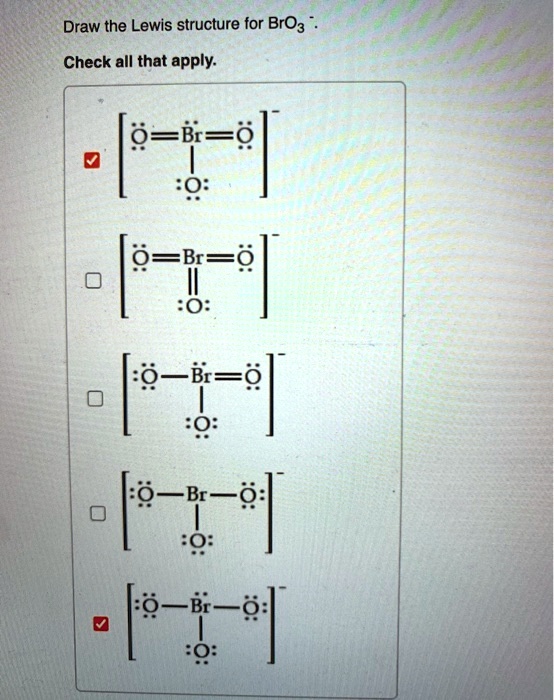
SOLVED Draw the Lewis structure for BrO3 Check all that apply 2 B=e B
First, we determine the total number of valence electrons in the molecule. Calculate the total number of valence electrons. Indicate the total number of valence electrons b. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. How many total equivalent likely resonance structures exist for bro3 ?? Breaking.
Pin on Latest
So, total valence electrons will be: Give the name of the ion. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms #4 minimize formal charges by converting lone pairs of the atoms, and try to get a stable lewis structure #5 repeat.
BrO3 Lewis Structure (Bromate Ion) YouTube
So, total valence electrons will be: Web chemistry chemistry questions and answers draw the lewis structure for the bromate ion (bro3−)with minimized formal charges. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet is already completed on central atom) #5 convert lone pair and.
Bro3 Lewis Structure
Web a) draw all the possible lewis structures for the following ions: Using formal charges to determine how many bonds to make, a different perspective. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web this problem has been solved! While selecting the atom, always put the least.
BrO3 Lewis Structure How to Draw the Lewis Structure for BrO3 YouTube
Indicate the total number of valence electrons b. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. So, total valence electrons will be: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. How many total equivalent likely resonance structures exist for.
Draw The Lewis Structure For Bro3- And this is an anion that carries one negative charge. 7 + 3 (6) + 1= 26 valence electrons. Draw the lewis structure for bro3 check all that apply. Calculate the total number of valence electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
This problem has been solved! Describe the preferred lewis structure. For the hbro3 structure use the periodic table to find the total number of valence electrons for the hbro3 molecule. Web this problem has been solved!
Calculate The Total Number Of Valence Electrons.
Here’s the best way to solve it. 7 in a lewis structure for a neutral molecule, the. Drawing lewis structures for bf3, pf3 and brf3; You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
First, We Determine The Total Number Of Valence Electrons In The Molecule.
Give the name of the ion. Using formal charges to determine how many bonds to make, a different perspective. How many total equivalent likely resonance structures exist for bro3 ?? Web drawing lewis structures for molecules with one central atom:
Web A) Draw All The Possible Lewis Structures For The Following Ions:
Indicate the total number of valence electrons b. Web chemistry chemistry questions and answers draw the lewis structure for the bromate ion (bro3−)with minimized formal charges. Different factors can affect the polarity of the molecule or atom. 2.7m views 11 years ago.