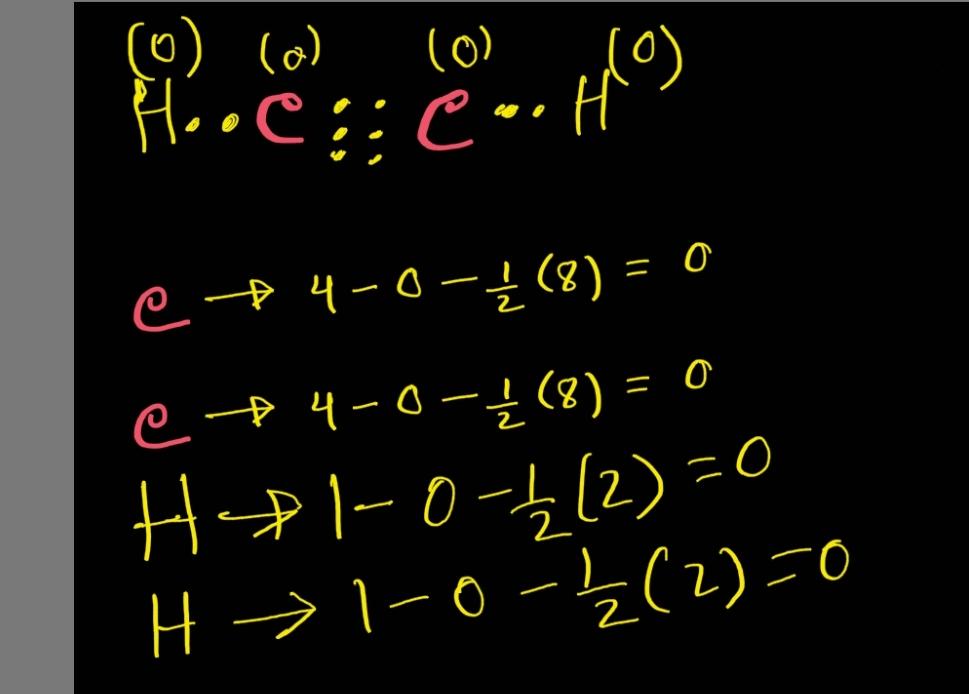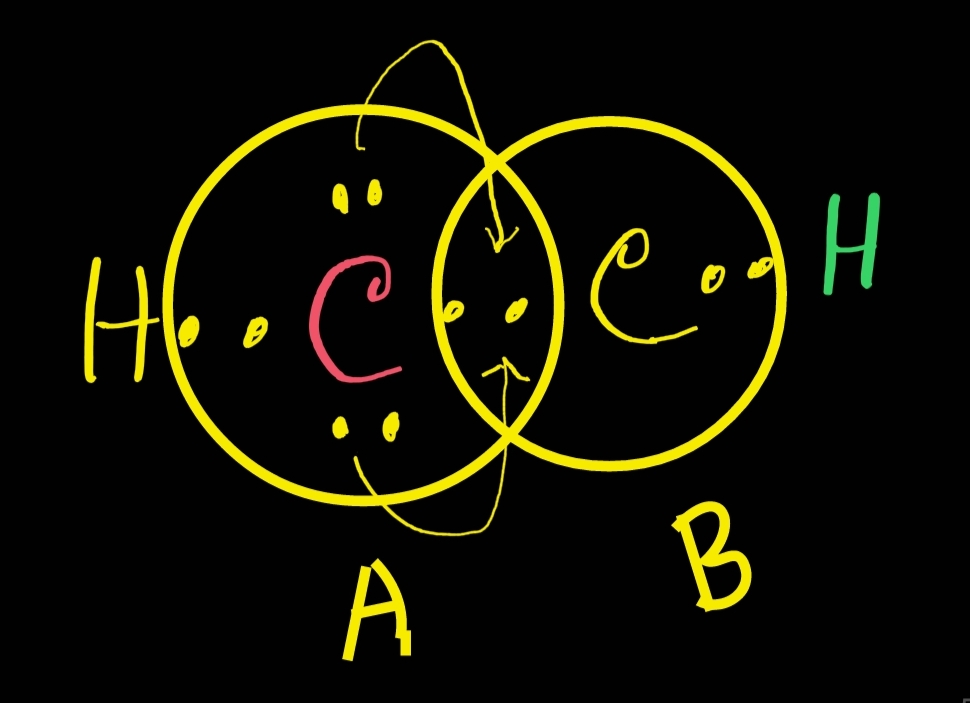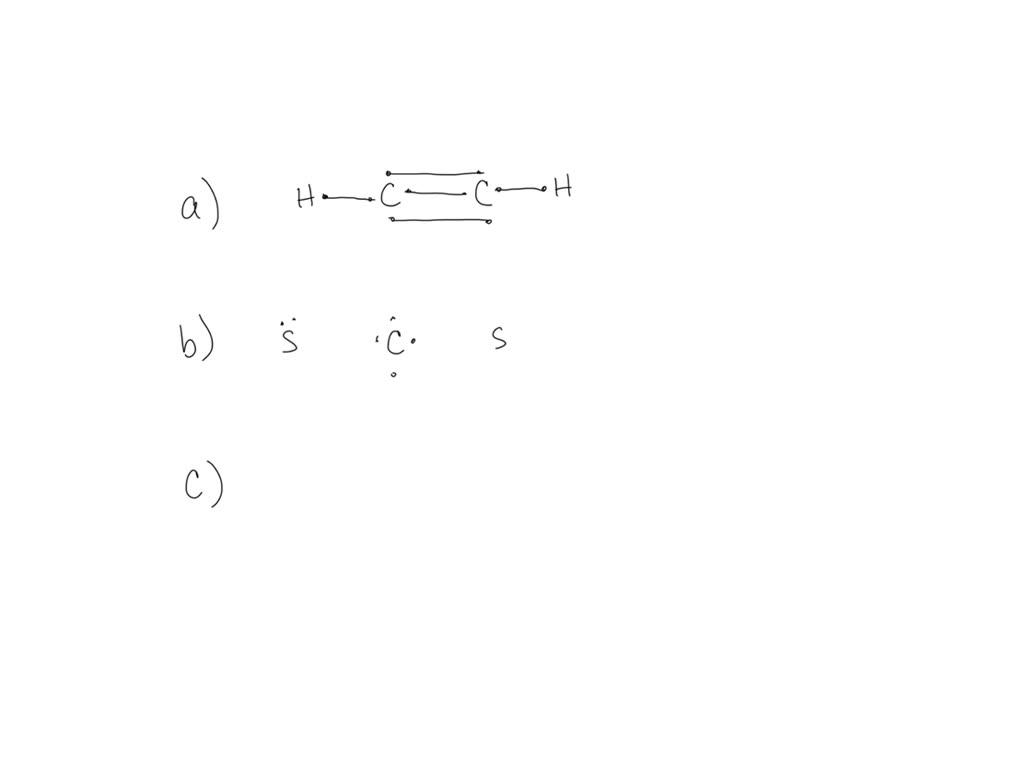Draw The Lewis Structure For Hcch
Draw The Lewis Structure For Hcch - Write the lewis structure for ch3coh and determine whether the molecule is polar. No terminal atoms capable of accepting electrons Draw lewis structures depicting the bonding in simple molecules. To do this, we add up the valence electrons of each. In this section , we will explore various aspects of the hcch lewis structure, including its resonance , shape, formal charge , angle, octet rule , valence electrons, and hybridization.
Find out the total number of valence electrons of all atoms.valence electrons in h = 1 electron. Web acetylene is used as fuel in some welding torches. In the lewis structure, the arrangement of atoms for acetylene is hcch. Remember that h is never a central atom: No terminal atoms capable of accepting electrons; Web chemistry questions and answers. Hydrogen atoms only need two electrons for a full outer shell.
HCCH Lewis structure ,Valence Electrons, Formal Charge
There are a total of 10 valence electrons for the hcch lewis structure. The lewis structures for h3cch3, h2cch2, and hcch. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. Web write lewis symbols for neutral atoms and ions. Include all hydrogen atoms and nonbonding electrons. No terminal.
HCCH Lewis structure ,Valence Electrons, Formal Charge
In this section , we will explore various aspects of the hcch lewis structure, including its resonance , shape, formal charge , angle, octet rule , valence electrons, and hybridization. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Where needed, distribute electrons to the terminal atoms: To.
SOLVEDDraw the Lewis structures for each of the following molecules or
Web write lewis symbols for neutral atoms and ions. Lewis structures are diagrams that show the arrangement of atoms and electrons in a molecule. To draw the lewis structure,. Part b draw the lewis structure for cs2 (where c is the central atom). Carbon (c) has 4 valence electrons, and hydrogen (h) has 1 valence.
PPT Drawing Lewis Structures PowerPoint Presentation, free download
Part b draw the lewis structure for cs2 (where c is the central atom). No terminal atoms capable of accepting electrons; In hcch, there are two carbon atoms and two hydrogen atoms. Web write lewis symbols for neutral atoms and ions. Remember that h is never a central atom: Since there are two carbon atoms.
HCCH Lewis structure ,Valence Electrons, Formal Charge
Include all hydrogen atoms and all lone pairs of electrons. No terminal atoms capable of accepting electrons; Where needed, distribute electrons to the terminal atoms: For the c2h2 structure use the periodic table to find the total number of valence electrons. Η ο h c c h h 100. Six electrons placed on n; Web.
HCCH Lewis Structure How to Draw the Lewis Structure for the HCCH
Where needed, distribute electrons to the terminal atoms: The lewis structures for h3cch3, h2cch2, and hcch. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Web for the hcch lewis structure you'll.
14+ Hcch Lewis Structure Robhosking Diagram
The lewis structures for h3cch3, h2cch2, and hcch. In the lewis structure, the arrangement of atoms for acetylene is hcch. Choose the central atom and draw the bond line structure.the central atom in hcch is c. Web to draw the lewis structure for hcch (ethyne), follow these steps: Start by determining the total number of.
Draw the Lewis structure for HCCH.Draw the molecule b… SolvedLib
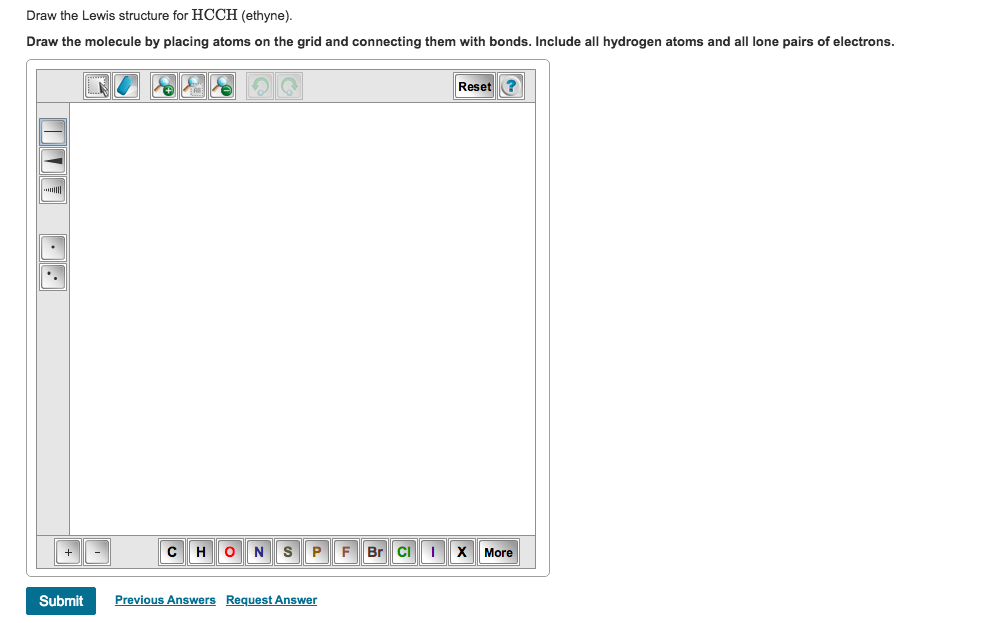
Draw the molecule by placing atoms. Six electrons placed on n; Is it a, b, c or d? Hydrogen atoms only need two electrons for a full outer shell. Web draw the lewis structure of hcch and then determine its electron domain and molecular geometries. Draw the molecule by placing atoms on the grid and.
Draw the Lewis structure for HCCH (ethyne) Draw the … SolvedLib
(note that c is the central atom.) Carbon (c) has 4 valence electrons, and hydrogen (h) has 1 valence electron. Where needed, distribute electrons to the terminal atoms: Web part a draw the lewis structure for hcch (ethyne). Remember that h is never a central atom: Thus far in this chapter, we have discussed the.
Solved Draw the Lewis structure for HCCH (ethyne). Draw the
C2h4 (whose skeletal structure is h2cch2) e. We have discussed the various types of bonds that form between atoms. For the c2h2 structure use the periodic table to find the total number of valence electrons. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely lewis. Web.
Draw The Lewis Structure For Hcch This problem has been solved! Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Six electrons placed on n; Draw the lewis structure for hcch (ethyne). Write the lewis structure for ch3coh and determine whether the molecule is polar.
Remember That H Is Never A Central Atom:
No terminal atoms capable of accepting electrons; Web acetylene is used as fuel in some welding torches. Web chemistry chemistry questions and answers 98. Draw the molecule by placing atoms on the grid and connecting them with bonds.
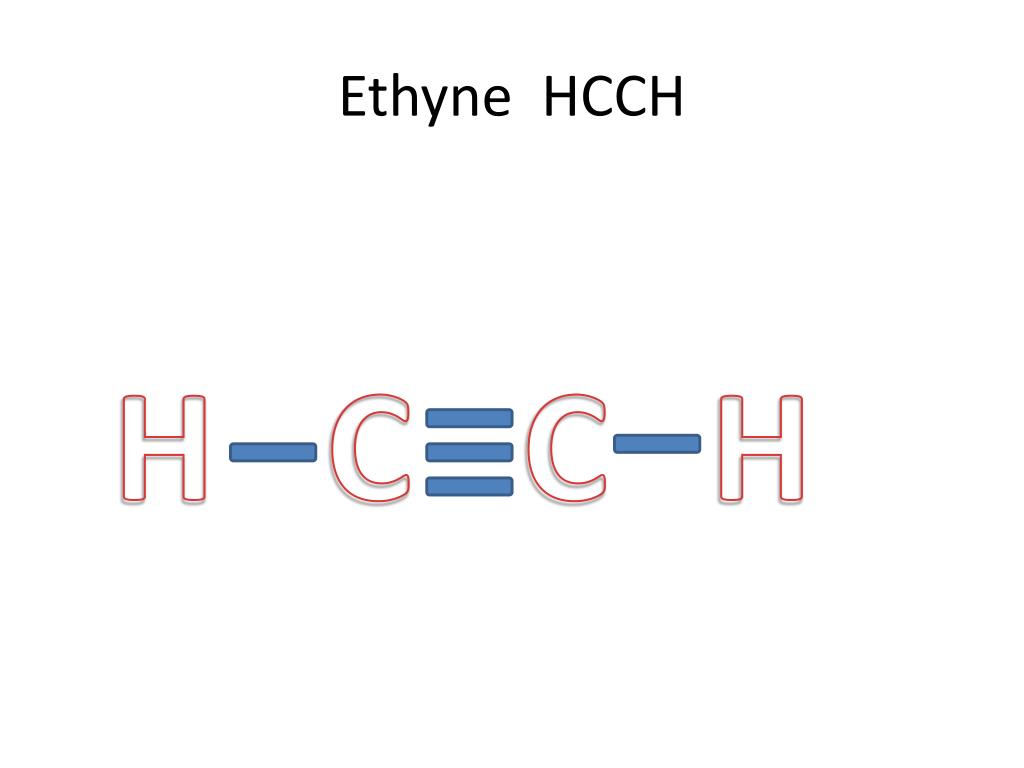
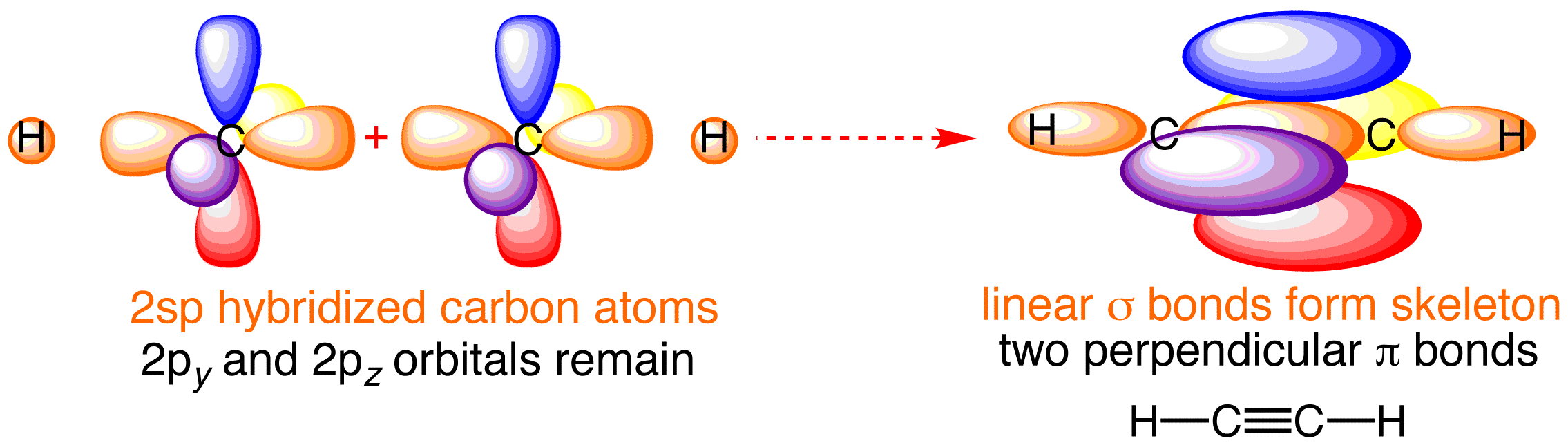
Web For The Hcch Lewis Structure You'll Need To Form A Triple Bond Between The Two Carbon Atoms.
Where needed, distribute electrons to the terminal atoms: These bonds involve the sharing or transfer of valence shell electrons between atoms. Η ο h c c h h 100. Draw the lewis structure for cocl2.
In This Section , We Will Explore Various Aspects Of The Hcch Lewis Structure, Including Its Resonance , Shape, Formal Charge , Angle, Octet Rule , Valence Electrons, And Hybridization.
Six electrons placed on n; Include all hydrogen atoms and nonbonding electrons. The lewis structure for acetylene contains: Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions.
Web To Draw The Lewis Structure For Hcch ( Acetylene ), Follow The Below Steps:
No terminal atoms capable of accepting electrons; Is it a, b, c or d? Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Note that h and f can only form one bond, and are always on the periphery rather than the central atom.