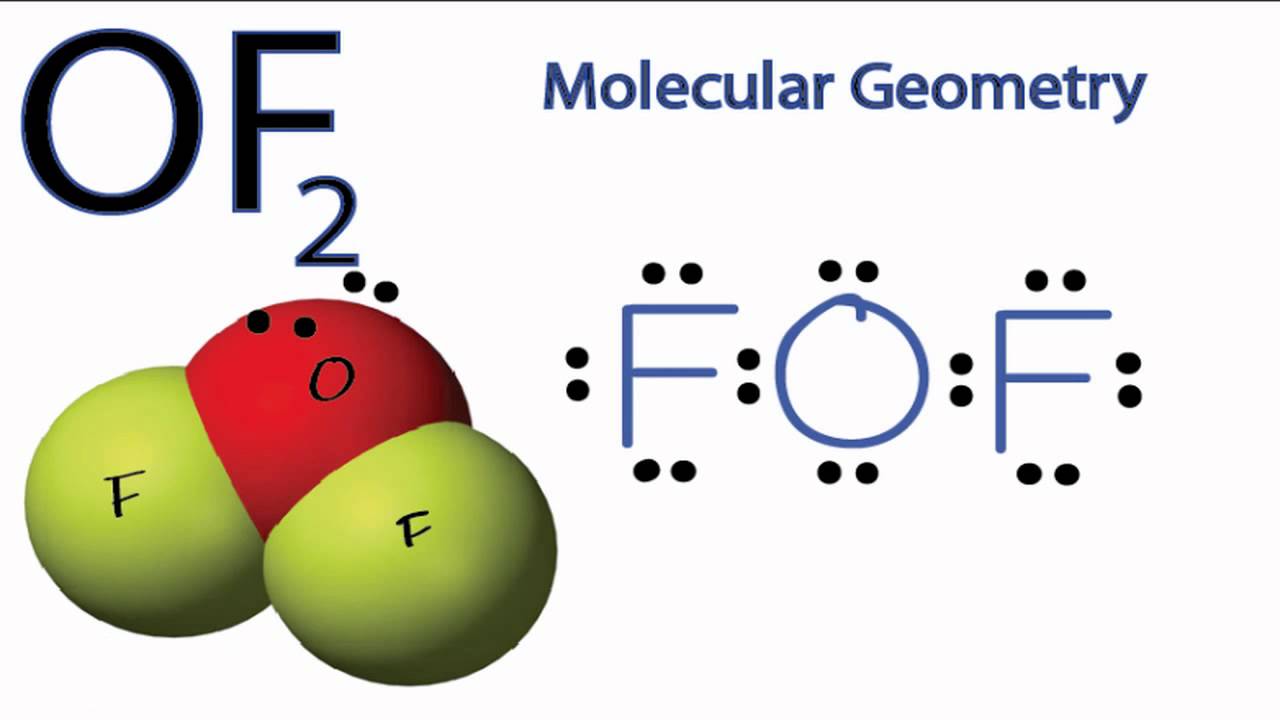
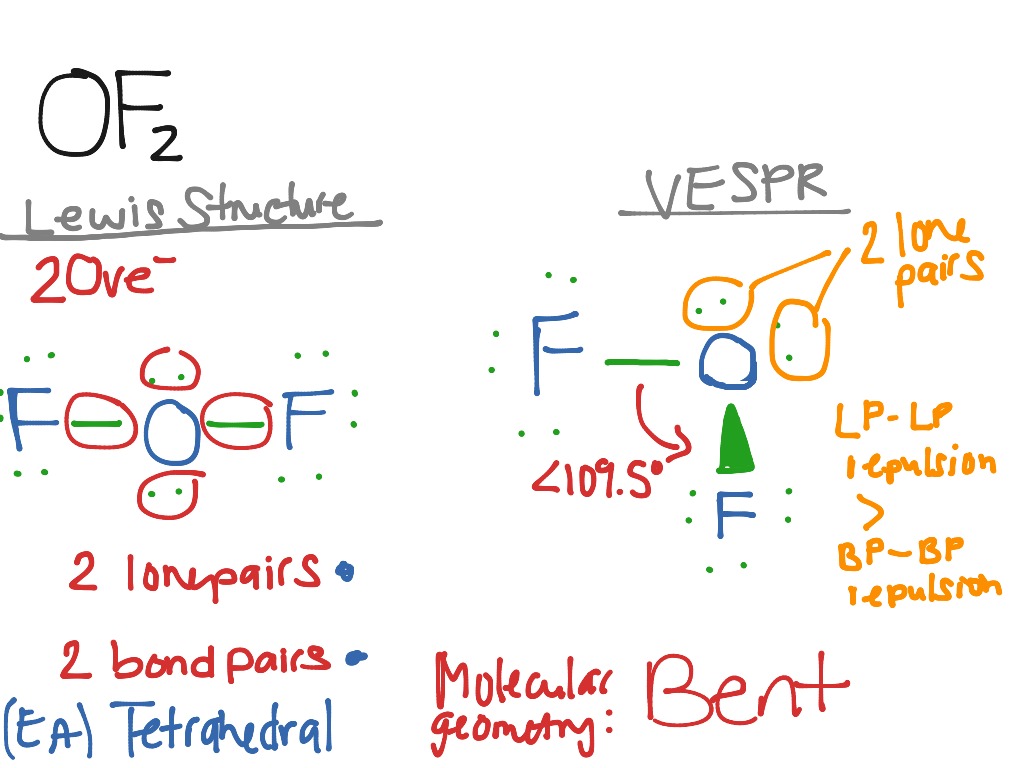
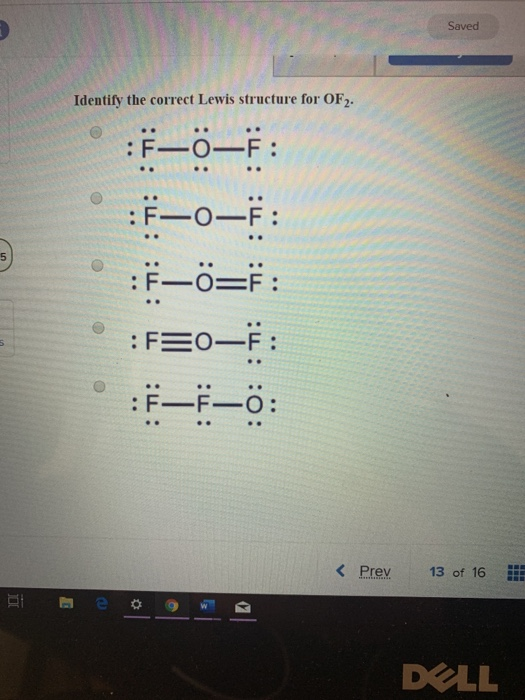
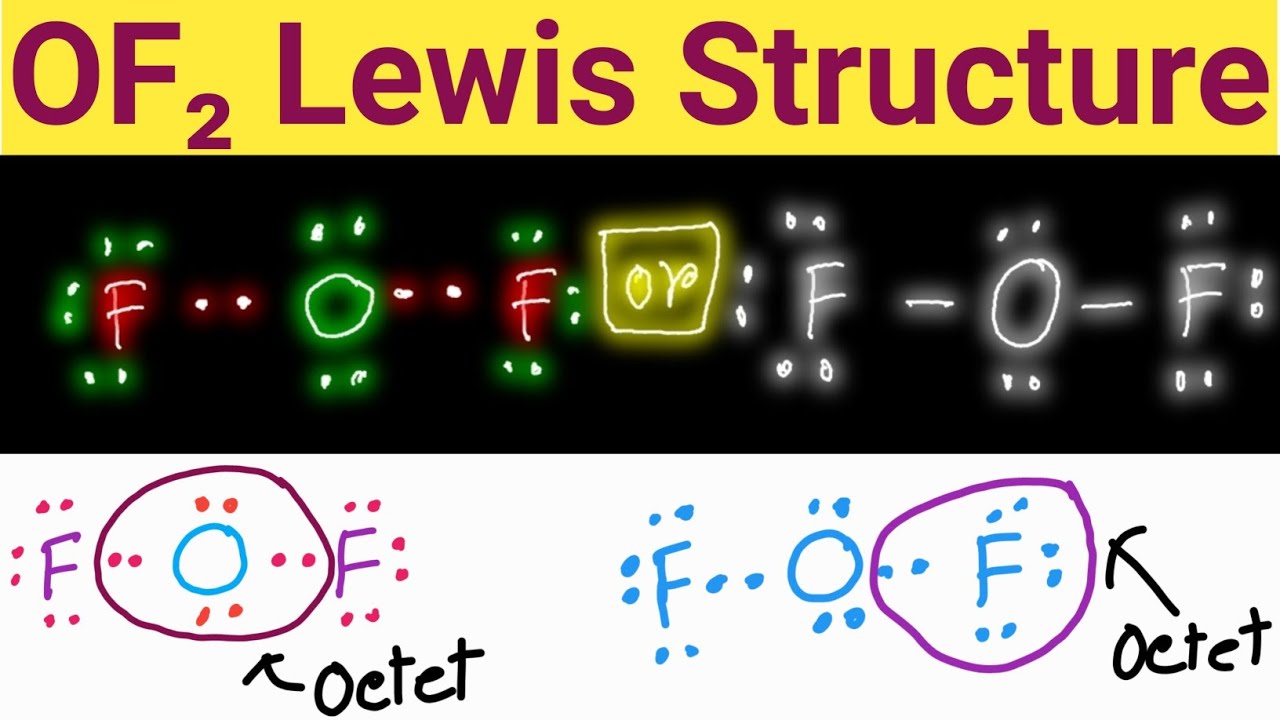
Draw The Lewis Structure For Of2
Draw The Lewis Structure For Of2 - Find the total valence electrons in of2 molecule in order to find the total valence electrons in of2 (oxygen difluoride) molecule, first of all you should know the valence electrons present in oxygen atom as well as fluorine atom. Web follow these simple steps to draw lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Draw lewis structures depicting the bonding in simple molecules. Web the lewis structure of xef 2 shows two bonding pairs and three lone pairs of electrons around the xe atom:
It is a chemical formula for oxygen difluoride. Determine the total number of valence (outer shell) electrons. Web answer to solved draw the lewis structure of of2, then answer A) how many bonding pairs of electrons are in the lewis structure? Find the total valence electrons in of2 molecule in order to find the total valence electrons in of2 (oxygen difluoride) molecule, first of all you should know the valence electrons present in oxygen atom as well as fluorine atom. We place three lone pairs of electrons around each f atom, accounting for 36 electrons. Web lewis structure for of.
OF2 Molecular Geometry YouTube
Web steps of drawing of2 lewis structure step 1: A) how many bonding pairs of electrons are in the lewis structure? Web write lewis symbols for neutral atoms and ions. Web of2, aka oxygen difluoride, is a molecular compound.oxygen brings 6 electrons, each fluorine brings 7 electrons, that's 20 electrons total.that's enough for. Here, the.
How to Draw a Lewis Structure
[ select] b) how many lone pairs of electrons are present on. For the of2 structure use the periodic table to find the total number of valence electrons for. Two electrons remain, and this lone pair is placed on the xe atom: Placing the least electronegative atom in the center fluorine is the most electronegative.
OF2 Lewis Structure How to Draw the Lewis Structure for OF2 YouTube
Determine the total number of valence electrons by adding up the valence electrons of all the atoms in the molecule. Web 6 steps to draw the lewis structure of of2 step #1: Web follow these simple steps to draw lewis dot structures: Web answer to solved draw the lewis structure of of2, then answer [.
[Solved] Draw a Lewis structure for each molecular compound a. OF2 b
2f2 + 2naoh ——> of2 + 2naf + h2o. Web 6 steps to draw the lewis structure of of2 step #1: Web hey guys, in this video we are going to learn about the lewis structure of of2. Breaking the octet rule ; Web how to draw lewis structure for of2? Draw the atoms on.
OF2 lewis structure, molecular geometry, hybridization and bond angle
Draw the atoms on paper and put dots around them to represent valence electrons of the atom. The equation for the preparation of oxygen difluoride: Breaking the octet rule ; If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Web the lewis structure of xef 2 shows.
OF2 Science, Chemistry, VESPR ShowMe
Find the total valence electrons in of2 molecule in order to find the total valence electrons in of2 (oxygen difluoride) molecule, first of all you should know the valence electrons present in oxygen atom as well as fluorine atom. A) how many bonding pairs of electrons are in the lewis structure? Determine the total number.
[Solved] Draw a Lewis structure for each molecular compound a. OF2 b
Web write lewis symbols for neutral atoms and ions. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail. 2f2 + 2naoh ——> of2 + 2naf + h2o. While selecting the atom, always put.
Drawing the Lewis Structure of OF2 (Oxygen Difluride) YouTube
Web follow these simple steps to draw lewis dot structures: Determine the total number of valence (outer shell) electrons. Web of2 lewis structure, molecular geometry, hybridization, polarity, and mo diagram. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Web of2, aka oxygen difluoride, is a molecular.
Solved Saved Identify the correct Lewis structure for OF2.
For the of2 structure use the periodic table to find the total number of valence electrons for the of2. Here, the given molecule is of2 (oxygen difluoride). Oxygen difluoride or of2 is a chemical compound formed by the reaction between halogen fluorine and dilute aqueous solution of naoh ( sodium hydroxide ). Draw the atoms.
OF2 Lewis Structure Lewis Dot Structure for OF2 Oxygen Difluoride
Web the lewis structure of xef 2 shows two bonding pairs and three lone pairs of electrons around the xe atom: Web hey guys, in this video we are going to learn about the lewis structure of of2. Drawing lewis structures for bf3, pf3 and brf3; We place three lone pairs of electrons around each.
Draw The Lewis Structure For Of2 For the of2 structure use the periodic table to find the total number of valence electrons for the of2. We place three lone pairs of electrons around each f atom, accounting for 36 electrons. A) how many bonding pairs of electrons are in the lewis structure? Web hey guys, in this video we are going to learn about the lewis structure of of2. This widget gets the lewis structure of chemical compounds.
Following Are The Steps To Follow To Draw The Lewis.
We place three lone pairs of electrons around each f atom, accounting for 36 electrons. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail. Web of2 lewis structure, molecular geometry, hybridization, polarity, and mo diagram. (oxygen difluroide) we draw lewis structures to predict:
Web The Lewis Structure Of Xef 2 Shows Two Bonding Pairs And Three Lone Pairs Of Electrons Around The Xe Atom:
For the of2 structure use the periodic table to find the total number of valence electrons for. A) how many bonding pairs of electrons are in the lewis structure? Two electrons remain, and this lone pair is placed on the xe atom: Thus far, we have discussed the various types of bonds that form between atoms and/or ions.
Determine The Total Number Of Valence Electrons By Adding Up The Valence Electrons Of All The Atoms In The Molecule.
Web how to draw lewis structure for of2? For the of2 structure use the periodic table to find the total number of valence electrons for the of2. Draw lewis structures depicting the bonding in simple molecules. Determine the total number of valence (outer shell) electrons.
Web Lewis Structure For Of.
The sum of the valence electrons is 5 (from n) + 6 (from o) = 11. Web of2, aka oxygen difluoride, is a molecular compound.oxygen brings 6 electrons, each fluorine brings 7 electrons, that's 20 electrons total.that's enough for. [ select] b) how many lone pairs of electrons are present on. Web use these steps to correctly draw the of 2 lewis structure: