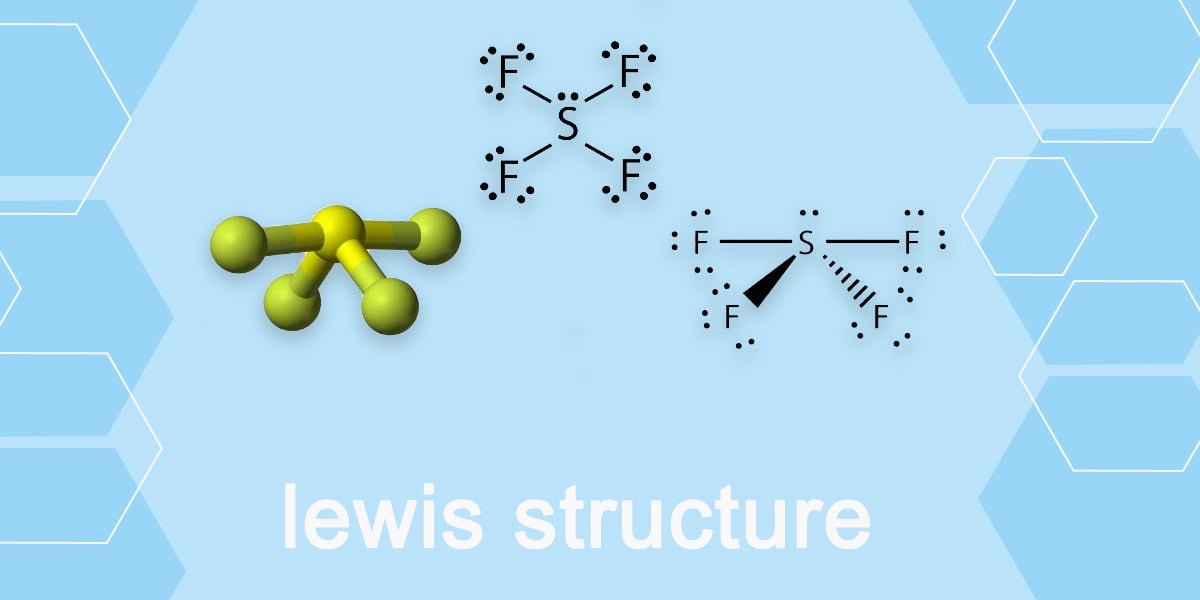
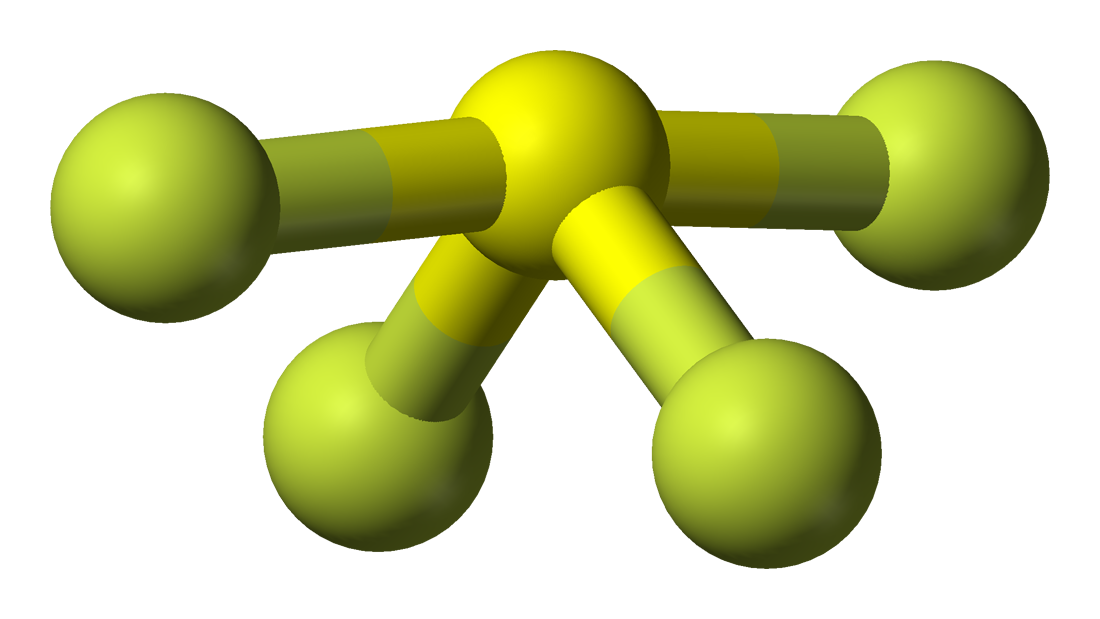
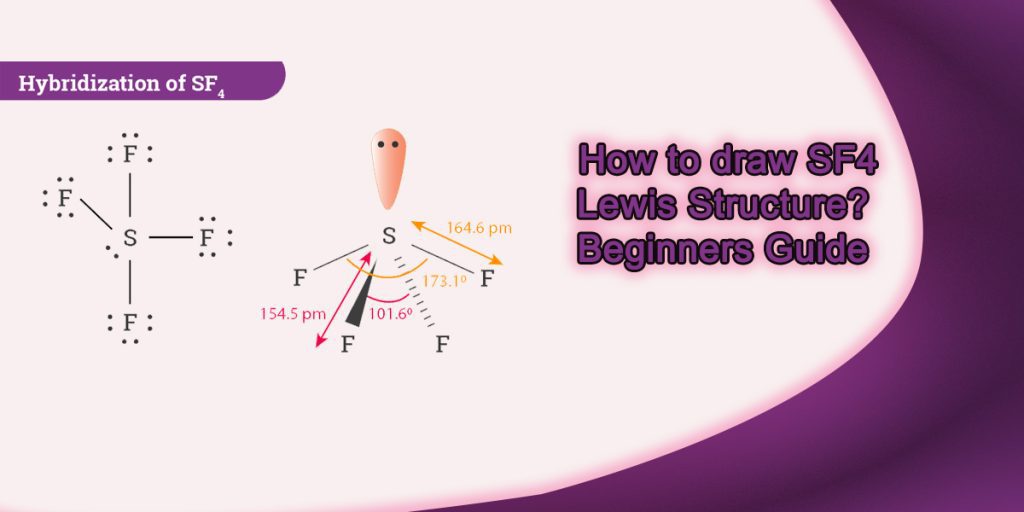
Draw The Lewis Structure For Sf4
Draw The Lewis Structure For Sf4 - Determine the total number of valence electrons by adding up the valence electrons of all atoms in the molecule. Web how to draw sf4 lewis structure? Web science chemistry chemistry questions and answers draw the lewis structure for sf4 in the window below and then answer the questions that follow. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Fluorine is in group 17, so each fluorine atom contributes 7 valence electrons.
Web draw the lewis dot structure for sf4 and determine the hybridization of the s atom and the f atoms. I also go over formal charge, hybridization, shape and bond angle. Sp3d, 1 od.sp3.0 o e. For the sf4 structure use the periodic table to find the total number of valence electrons. Watch the video and see if you missed any steps or information. It is helpful if you: Web science chemistry chemistry questions and answers draw the lewis structure for sf4.
How to draw Sf4 Lewis Structure? Beginners Guide
We'll put the s, the least electronegative, at the center. This problem has been solved! In this lewis structure of sf 4, center sulfur atom has made four single bonds with four fluorine atoms. Calculate the total number of valence electrons. Fluorine is in group 17, so each fluorine atom contributes 7 valence electrons. It.
SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
Calculate the total number of valence electrons. On the periodic table, 6 valence electrons for sulfur, 7 for fluorine but we have 4 fluorines; We'll put the fluorines around it, all four of them. Web there are a total of 34 valence electrons in the lewis structure for sf4. It is helpful if you: Identify.
SF4 Molecular Geometry, Lewis Structure, Bond Angles and Polarity
Determine the total number of valence electrons by adding up the valence electrons of all atoms in the molecule. It is helpful if you: Find the total valence electrons in sf4 molecule in order to find the total valence electrons in sf4 molecule, first of all you should know the valence electrons present in. Draw.
SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube
Watch the video and see if you missed any steps or information. On the periodic table, 6 valence electrons for sulfur, 7 for fluorine but we have 4 fluorines; Web chemistry chemistry questions and answers the lewis structure for sf4 is shown. You'll get a detailed solution from a subject matter expert that helps you.
SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
For sf4, sulfur has 6 valence electrons, and each fluorine atom has 7 valence electrons, so the total is 6 + 4 (7) = 34 electrons. For the lewis structure for sf4 you should take formal charges into account to find the best lewis structure for the molecule. In this lewis structure of sf 4,.
SF4 Lewis Dot structureHow to draw the Lewis structure for SF4. neet
This problem has been solved! For the sf4 structure use the periodic table to find the total number of valence electrons. The central atom in this compound is sulfur. For sf4, sulfur has 6 valence electrons, and each fluorine atom has 7 valence electrons, so the total is 6 + 4 (7) = 34 electrons..
SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube
For the sf4 structure use the periodic table to find the total number of valence electrons. It is helpful if you: Web i quickly take you through how to draw the lewis structure of sf4, sulfur tetrafluoride. This problem has been solved! Determine the total number of valence electrons to begin, count the total number.
How to draw SF4 Lewis Structure? Science Education and Tutorials
This problem has been solved! Web how to draw sf4 lewis structure? Draw (on paper) a lewis structure for sf4 and answer the following questions based on your drawing. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Each bond, two valence electrons. This problem has been solved!.
How to draw SF4 Lewis Structure? Science Education and Tutorials
Web draw the lewis structure of sf4 showing all lone pairs. Web drawing lewis structures for molecules with one central atom: I also go over formal charge, hybridization, shape and bond angle. Calculate the total number of valence electrons. Web draw the lewis dot structure for sf4 and determine the hybridization of the s atom.
How to draw Sf4 Lewis Structure? Beginners Guide
Next, we put bonds between atoms. Web i quickly take you through how to draw the lewis structure of sf4, sulfur tetrafluoride. #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. The following procedure will give you the correct lewis.
Draw The Lewis Structure For Sf4 The number of lone pairs =. The central atom in this compound is sulfur. Individually speaking, valence electrons of sulfur = 6 valence electrons of fluorine = 7 Sp3d, 1 od.sp3.0 o e. Web there are a total of 34 valence electrons in the lewis structure for sf4.
Web I Quickly Take You Through How To Draw The Lewis Structure Of Sf4, Sulfur Tetrafluoride.
0 this problem has been solved! We'll put the fluorines around it, all four of them. Calculate the total number of valence electrons. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom.
Web Science Chemistry Chemistry Questions And Answers Draw The Lewis Structure For Sf4 In The Window Below And Then Answer The Questions That Follow.
Web draw the lewis dot structure for sf4 and determine the hybridization of the s atom and the f atoms. While selecting the atom, always put the least electronegative atom at the center. It is helpful if you: For the central sulfur atom:
Sp3D, 1 Od.sp3.0 O E.
Web to draw the structure of sf4 we will need to know the total number of valence electrons that exist in sf4. First, we arrange the atoms, then distribute the valence electrons, and finally, place them around the atoms to form bonds and lone pairs. Try to draw the sf 4 lewis structure before watching the video. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Web There Are A Total Of 34 Valence Electrons In The Lewis Structure For Sf4.
Web to draw the lewis structure for sf4, we start by determining the total number of valence electrons. Watch the video and see if you missed any steps or information. Web let's do the sf4 lewis structure. Web how to draw sf4 lewis structure?