Draw The Lewis Structure For Xef2
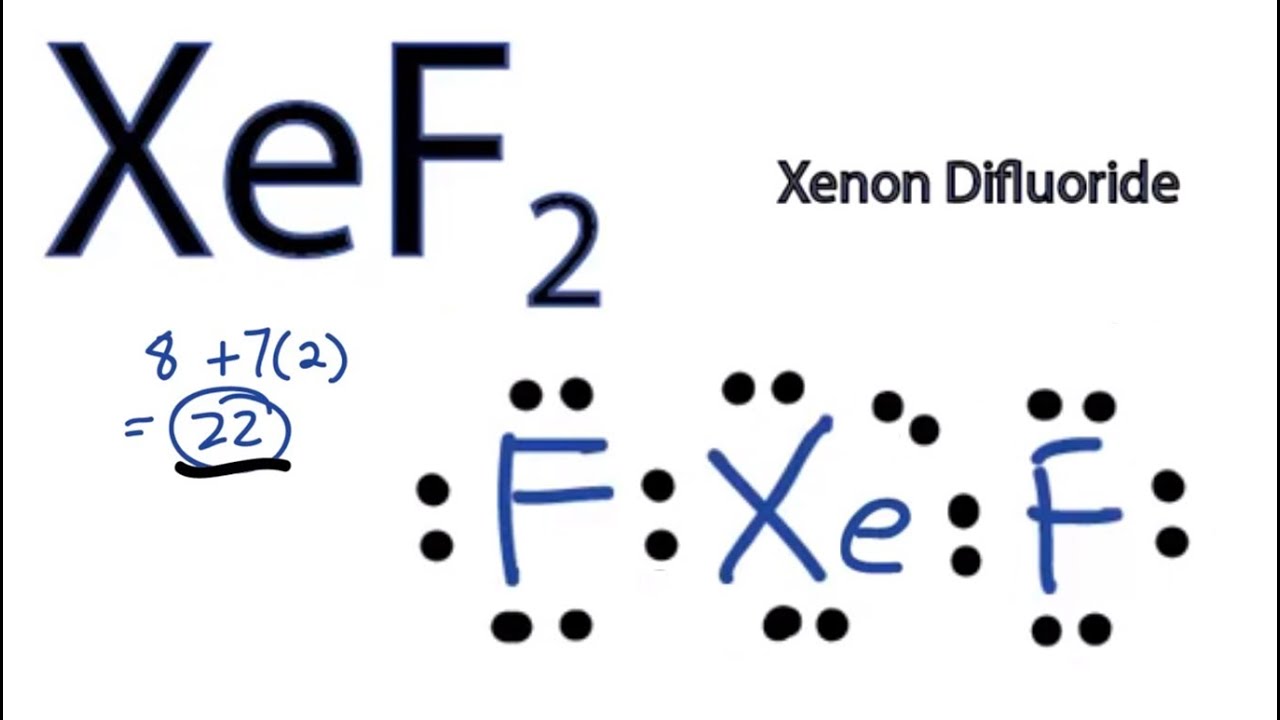
Draw The Lewis Structure For Xef2 - Web to draw the xef2 lewis structure, follow these steps: C) what is the molecular shape of this molecule? We use dots to represent outer shell electrons and lines to represent the bond type. Determine the total number of valence electrons: Web lewis dot structure of xef2 (xenon difluoride) 97,881 views.
Xenon has eight valence electrons, and each fluorine atom has seven valence electrons, so the total number of valence electrons is 22. Draw the lewis structure for xef2 a) how many groups (atoms and lone pairs) surround the central oxygen? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Out of the 5 electron pairs, 2 are bond pairs while there are 3 lone pairs present on the xe atom. Xef2 lewis structure involves 1 atom of xenon and 2 atoms of fluorine. Determine the total number of valence electrons in xef2 by adding the valence electrons of each atom. It is one of those rare compounds which involve noble gases despite their strong stability.
Molecular geometry of XeF2 [with video and free study guide]
Xef2 lewis structure involves 1 atom of xenon and 2 atoms of fluorine. Web to draw the xef2 lewis structure, follow these steps: It is one of those rare compounds which involve noble gases despite their strong stability. Sp4 a draw a lewis structure for the ammonia molecule (nh3) and complete the following statement: Web.
The Lewis Structure Of Xef2 Understanding The Bonding Of Xenon
What is the electronic geometry of this molecule (look at atoms and lone pairs)? Xef2 lewis structure and its properties are illustrated in this article. As we know xenon lies in. For the xef2 structure use the periodic table to find the total number of valence electron. Out of the 5 electron pairs, 2 are.
The Lewis Structure Of Xef2 Understanding The Bonding Of Xenon
Drawing the lewis structure for xef2. Web by using the following steps, you can easily draw the lewis structure of xef 2: See an example of a molecule that violates the octet rule (xef₂) and learn how to draw its lewis diagram in this video. While selecting the atom, always put the least electronegative atom.
36+ Xef2 Lewis Structure Molecular Geometry Image GM
The lewis structure for xef 2 requires you to place more than 8 valence electrons on xe. I quickly take you through how to draw the lewis structure of xef2 (xenon difluoride). Sp4 a draw a lewis structure for the ammonia molecule (nh3) and complete the following statement: Web lewis structure is based on the.
XeF2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
Drawing the lewis structure for xef2. Web by using the following steps, you can easily draw the lewis structure of xef 2: Web chemistry chemistry questions and answers draw a lewis structure for xef2 and answer the following questions based on your drawing. Xenon (xe) is in group 18 of the periodic table and has.
[Solved] Identify the correct Lewis structure for XeF2. O F=Xe=F O
Web use these steps to correctly draw the xef 2 lewis structure: С p opy aste []* с chemdoodle is xef2 polar or nonpolar? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The central xenon atom a. Web science chemistry chemistry questions and answers draw the lewis.
XeF2 Lewis Structure How to Draw the Lewis Structure for XeF2 YouTube
Xenon (xe) can have more than 8. Web 5 steps to draw the lewis structure of xef2 step #1: There are a total of 5 electron pairs in this lewis structure. What is the electronic geometry of this molecule (look at atoms and lone pairs)? Xenon has eight valence electrons, and each fluorine atom has.
Xef2 Lewis Structure Lone Pairs Drawing Easy
Determine the total number of valence electrons: Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). This problem has been solved! Find the total valence electrons in xef2 molecule in order to find the total valence electrons in xef2 (xenon difluoride).
Hello Guys! Today we are going to look at the Lewis Structure of XeF2
Draw this vsepr structure next to the lewis structure. Xenon (xe) is in group 18 of the periodic table and has 8 valence electrons. Determine the total number of valence electrons in xef2 by adding the valence electrons of each atom. Knowing how many valence electrons there a. #1 first draw a rough sketch #2.
XeF2 Lewis Structure How to Draw the Lewis Structure for XeF2 (Xenon
Sp4 a draw a lewis structure for the ammonia molecule (nh3) and complete the following statement: #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail. Lewis diagram of xenon difluoride (xef₂) in some.
Draw The Lewis Structure For Xef2 Drawing the lewis structure for xef2. Web © 2023 google llc for the xef2 structure use the periodic table to find the total number of valence electrons for the xef2 molecule. Determine the total number of valence electrons in xef2 by adding the valence electrons of each atom. Sp4 a draw a lewis structure for the ammonia molecule (nh3) and complete the following statement: Xenon (xe) is in group 18 of the periodic table and has 8 valence electrons.
This Rule States That Every Molecule Should Have Eight Electrons In Its Outer Shell Of An Atom To Be Stable.
Web watch on steps of drawing xef2 lewis structure step 1: Knowing how many valence electrons there a. Here, the given molecule is xef2 (xenon difluoride). Xef2 lewis structure and its properties are illustrated in this article.
I Also Go Over Hybridization, Shape And Bond Angle.
Ii the number of lone pairs the number of single bonds the number of double bonds = 2. Web science chemistry chemistry questions and answers draw the lewis structure for xef2 in the window below and then answer the questions that follow. Xenon is an inert gas element. Determine the total number of valence electrons in xef2 by adding the valence electrons of each atom.
For The Xef2 Lewis Structure We First Count The.
Drawing the lewis structure for xef2. If there are more electrons than it, then that compound donates the electron. Out of the 5 electron pairs, 2 are bond pairs while there are 3 lone pairs present on the xe atom. Determine the total number of valence electrons:
This Problem Has Been Solved!
Web by using the following steps, you can easily draw the lewis structure of xef 2: #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail. Web lewis structure is based on the octet rule. Xenon (xe) is in group 18 of the periodic table and has 8 valence electrons.

![Molecular geometry of XeF2 [with video and free study guide]](https://aceorganicchem.com/chemistry/wp-content/uploads/2023/06/XeF2-lewis.jpg)