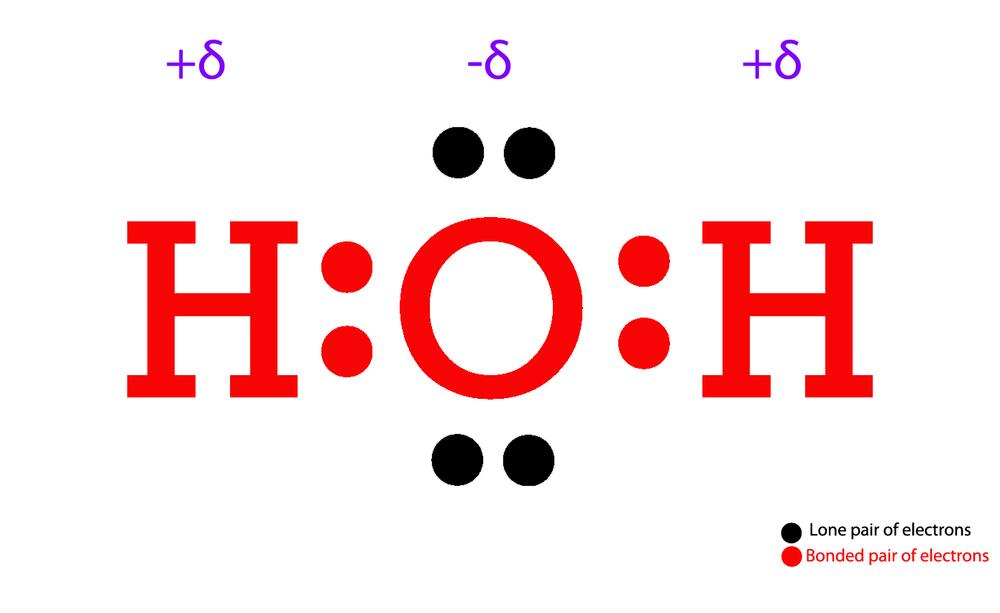
Draw The Lewis Structure Of H2O
Draw The Lewis Structure Of H2O - O has 6 valence electrons, and each h has one. Web = 8 valence electrons thus, h2o has a total of 8 valence electrons. In order to draw the lewis structure of h2o2, first of all you have to find the total number of valence electrons present in the h2o2 molecule. You must arrange 8 electrons in pairs so that o has 8 and each h has two electrons in its valence shell. Web 33k views 3 days ago.
Web how to draw lewis structure of h2o determine the total number of electrons in the oxygen and hydrogen atoms’ valence shells. Web how to draw lewis structure for h2o 1. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. O has 6 valence electrons, and each h has one. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. You must arrange 8 electrons in pairs so that o has 8 and each h has two electrons in its valence shell. Total electron pairs are calculated by dividing.
H2O Lewis structure and Molecular Geometry [No1 Best Explanation
A lewis structure may not give us a complete description of the molecular geometry. In order to draw the lewis structure of h2o2, first of all you have to find the total number of valence electrons present in the h2o2 molecule. Web may 22, 2023 by jay rana ready to learn how to draw the.
Lewis Dot Diagram For H2o Free Diagram For Student
Organic chemistry lewis structures and bonding lewis dot diagram. Look for the total valence electrons: Web here are the steps to draw the h2o lewis structure: Hydrogen is a group ia. A lewis structure may not give us a complete description of the molecular geometry. H2o lewis structure lewis structure for any molecule helps to.
Lewis Structure of Water H2O YouTube
Determine the total electron pairs in the form of lone pairs and bonds. Check the stability and minimize charges. (valence electrons are the number of electrons present in the outermost shell. Web here are the steps to draw the h2o lewis structure: Here, i have explained 6 simple steps to draw the lewis dot structure.
H2O Lewis Structure, Molecular Geometry, and Hybridization
It is four for one water (h2o) molecule according to the octet rule. H2o lewis structure lewis structure for any molecule helps to know the bonds formed in the structure and the electrons participating in the bond formation. Drawing the lewis structure for h 2 o when you are learning to draw lewis structures you.
H2O Lewis structure and Molecular Geometry [No1 Best Explanation
Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. The total electron pairs are calculated by. H2o lewis structure lewis structure for any molecule helps to know the bonds formed in the structure and the electrons participating in the bond formation. A simple notation used to represent valence electrons.
In this video we are going to learn about the Lewis structure of H2O
Determine the total electron pairs in the form of lone pairs and bonds. (valence electrons are the number of electrons present in the outermost shell. O has 6 valence electrons, and each h has one. Web how to draw lewis structure for h2o waterlewis structure: Web you can find a procedure for drawing lewis structures.
H2O Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Look for how many electrons are needed: How to draw lewis structure of h2o water (h2o) is a molecule composed of two hydrogen atoms bonded to a central oxygen atom. For h2o, there are 2 valence electrons for each hydrogen atom and 6 valence electrons for the oxygen atom, giving a total of 8 valence.
Draw Step By Step The Lewis Structure For Water (H2O)
First, we shall draw the lewis structure of h2o2. Web you can find a procedure for drawing lewis structures at this location. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web how to draw lewis structure of h2o determine the total number of electrons in the oxygen and hydrogen.
H2O Lewis structure and Molecular Geometry [No1 Best Explanation
Web h2o2 lewis structure. How to draw lewis structure of h2o water (h2o) is a molecule composed of two hydrogen atoms bonded to a central oxygen atom. Oxygen has six valence electrons, and hydrogen has one valence electron each, giving a total of eight valence electrons. Web drawing lewis structures for molecules with one central.
H2O Lewis Structure, Molecular Geometry, and Hybridization
With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Organic chemistry lewis structures and bonding lewis dot diagram. It is four for one water (h2o) molecule according to.
Draw The Lewis Structure Of H2O Still, it is helpful to visualize the overall skeletal structure of the molecule, its bonds, and its lone pairs. Organic chemistry lewis structures and bonding lewis dot diagram. I quickly take you through how to draw the lewis structure of water, h2o. For the h2o structure use the periodic table to find the total number of valence electrons for the h2o. Web here, we need to understand how the lewis structure is drawn for the h2o molecule:
Web Steps Of Drawing Lewis Structure Of H 2 O Find Total Number Of Electrons Of The Valance Shells Of Hydrogen Atoms And Oxygen Atom Total Electrons Pairs As Lone Pairs And Bonds Center Atom Selection Mark Lone Pairs On Atoms Mark Charges On Atoms If There Are.
Find the total number of. Calculate the total number of valence electrons. In order to draw the lewis structure of h2o2, first of all you have to find the total number of valence electrons present in the h2o2 molecule. Determine the total electron pairs in the form of lone pairs and bonds.
I Quickly Take You Through How To Draw The Lewis Structure Of Water, H2O.
Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. Shruti ramteke in this guide, we will learn how to draw the lewis structure of h2o, also known as water. Web 33k views 3 days ago. So, if you are ready to go with.
The Total Electron Pairs Are Calculated By.
Total electron pairs are calculated by dividing. We shall discuss the chemical bonding nature of h2o2 in this article. Look for how many electrons are needed: Group ia element hydrogen has.
Web The Structure On The Right Is The Lewis Electron Structure, Or Lewis Structure, For H 2 O.
The electrons that participate in bond formation are known as the bonding pair of electrons. Here, i have explained 6 simple steps to draw the lewis dot structure of h2o (along with images). It is eight to form a single h2o molecule. Web how to draw lewis structure for h2o 1.