Draw The Lewis Structure Of Of2
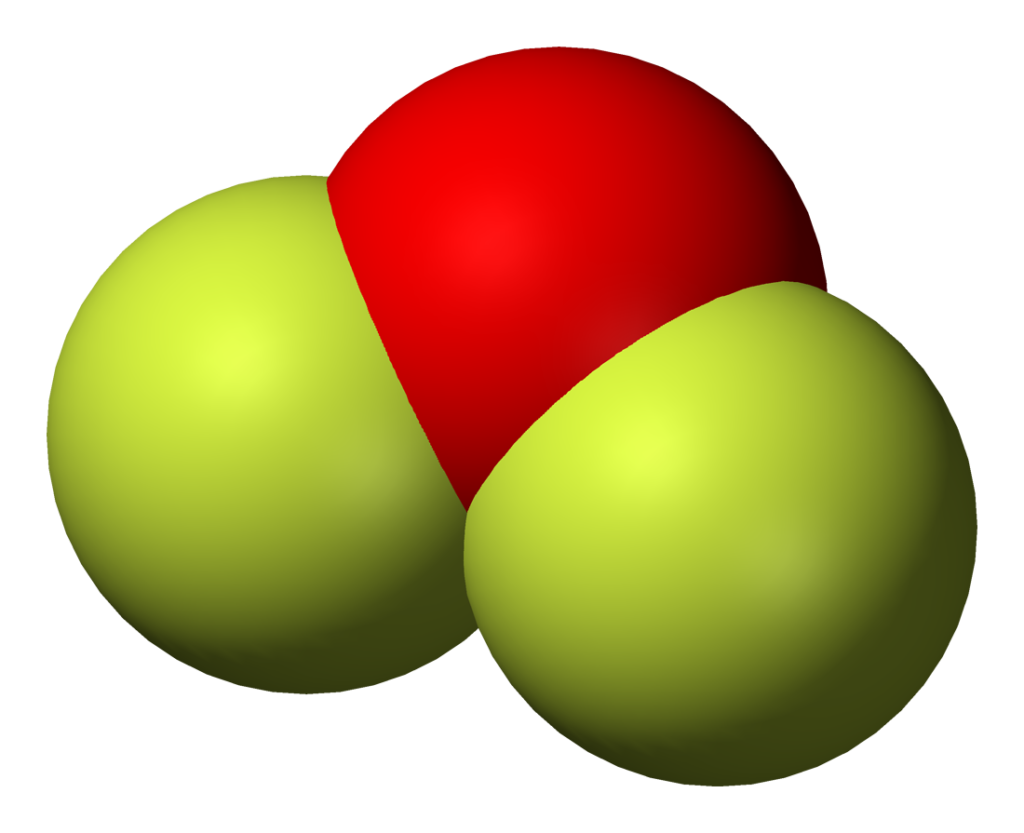
Draw The Lewis Structure Of Of2 - It is a chemical formula for oxygen difluoride. Web the of2 lewis structure consist of one oxygen atom as the central atom and two fluorine atom are present on the either side of the oxygen atom. Diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion. Counting valence electrons begin by counting the total valence electrons in the molecule. Web write lewis symbols for neutral atoms and ions.
For the of2 structure use the periodic table to find the total number of valence electrons for the of2. This widget gets the lewis structure of chemical compounds. Counting valence electrons begin by counting the total valence electrons in the molecule. Some students come to chem 101a having been taught to draw lewis structures with extra double bonds. Two (a pair of) valence electrons that are not used to form a covalent bond Understand the proper use of the octet rule to predict bonding in simple molecules. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail.
OF2 lewis structure, molecular geometry, hybridization and bond angle
Since fluorine is in period 2, it can fit a maximum of eight (8) electrons second energy level. Web draw the lewis dot structure for the hydrogen atom. Subtracting the number in step 1 from the number in step 2 gives you the number of electrons needed to. Sketch the skeletal diagram of the molecule.
OF2 Lewis Structure How to Draw the Lewis Structure for OF2 YouTube
Web hey guys, in this video we are going to learn about the lewis structure of of2. Subtracting the number in step 1 from the number in step 2 gives you the number of electrons needed to. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal.
OF2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
Counting valence electrons begin by counting the total valence electrons in the molecule. Web hey guys, in this video we are going to learn about the lewis structure of of2. Fluorine group vii, which means it has a total of seven (7. Web we can draw the lewis structure of any covalent molecule by following.
[Solved] Draw a Lewis structure for each molecular compound a. OF2 b
Web watch on steps of drawing of2 lewis structure step 1: Covalent bonds are formed when one electron from each atom forms an electron pair. (oxygen difluroide) we draw lewis structures to predict: Web how to draw lewis structure for of2 oxygen difluoridelewis structure: Find the total valence electrons in of2 molecule in order to.
Drawing the Lewis Structure of OF2 (Oxygen Difluride) YouTube
[ select] b) how many lone pairs of electrons are present on the central atom in the lewis structure? In this case, we can condense the last few steps, since not all of them apply. Draw the correct lewis structure of of2 and then use it to answer the questions below. A) how many bonding.
OF2 Molecular Geometry YouTube
Since fluorine is in period 2, it can fit a maximum of eight (8) electrons second energy level. Web 6 steps to draw the lewis structure of of2 step #1: This molecule is made up of one oxygen atom and two fluorine atoms. Get the free lewis structure finder widget for your website, blog, wordpress,.
OF2 Lewis Structure Lewis Dot Structure for OF2 Oxygen Difluoride
Web chemistry questions and answers. Web use these steps to correctly draw the of 2 lewis structure: Draw the correct lewis structure of of2 and then use it to answer the questions below. Draw lewis structures depicting the bonding in simple molecules. Find more chemistry widgets in wolfram|alpha. Web lewis structure for of. Web the.
Lewis Dot Structure for OF2 (Oxygen difluroide) YouTube
Web 6 steps to draw the lewis structure of of2 step #1: Here, the given molecule is of2 (oxygen difluoride). (oxygen difluroide) we draw lewis structures to predict: [ select] b) how many lone pairs of electrons are present on the central atom in the lewis structure? Diagram showing lone pairs and bonding pairs of.
Solved Draw the Lewis structure for OF2 and use it to
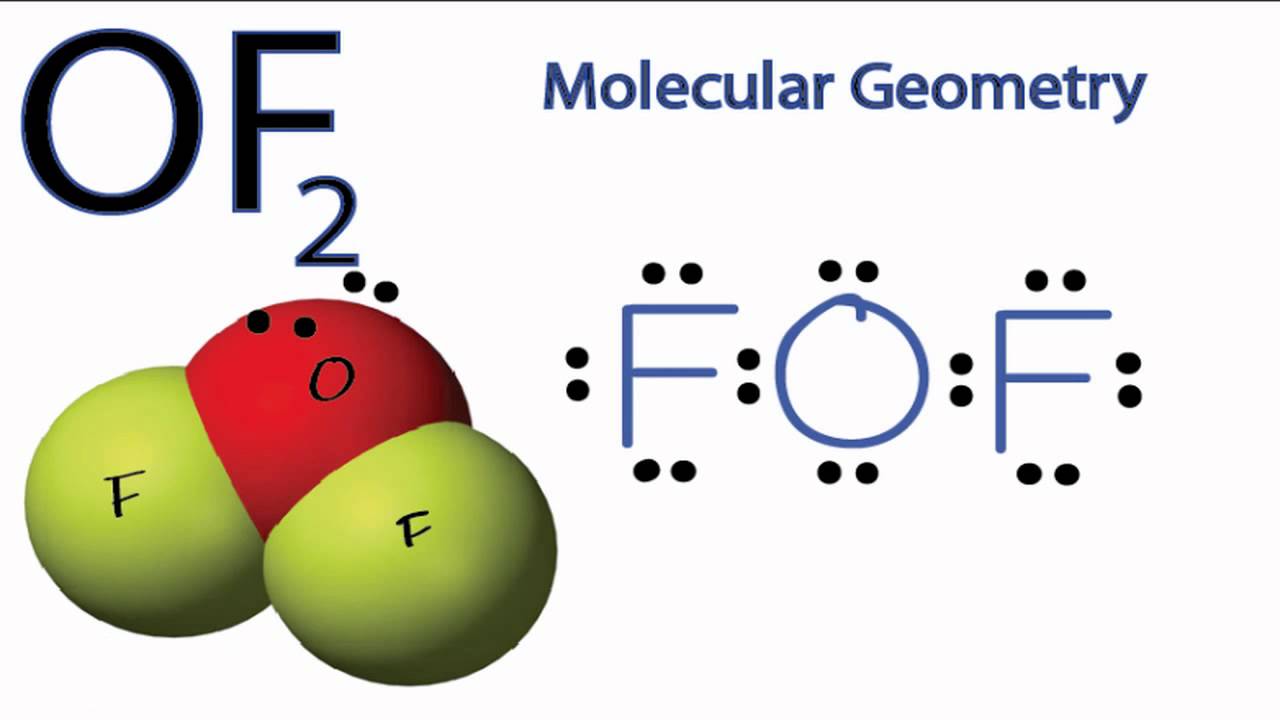
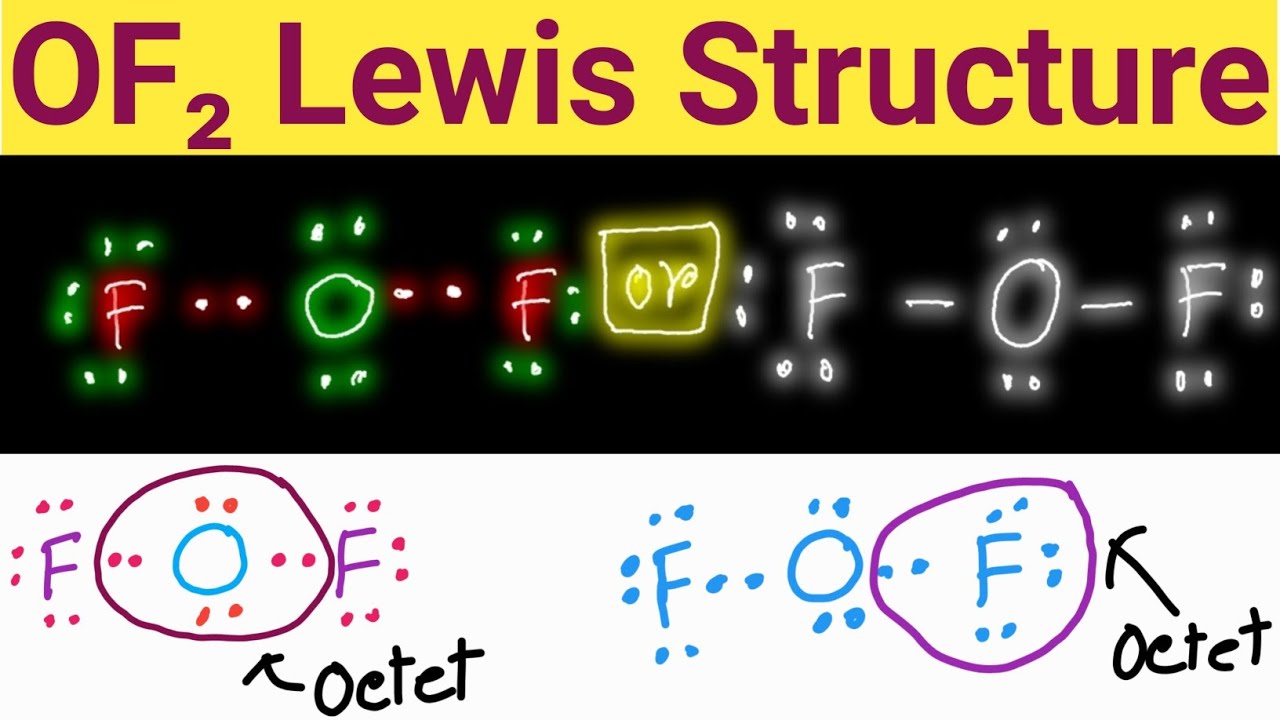
Web in the lewis structure of of2, both fluorine atoms share a single bond with the oxygen. Therefore, we recommend that when you draw a structure that satisfies the octet rule, you stop there without adding more bonds. Web the lewis structure of of2 contains two single bonds, with oxygen in the center, and two.
[Solved] draw the lewis dot structure for the following molecule/ion
Covalent bonds are formed when one electron from each atom forms an electron pair. Draw a skeleton joining the atoms by single bonds. The first and foremost step is to calculate the total number of. Identify the central atom now, we have to decipher the central atom in this molecule. Since fluorine is in period.
Draw The Lewis Structure Of Of2 Since hydrogen is in group i it has one (1) valence electron in its shell. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail. Draw a skeleton joining the atoms by single bonds. Web step 1: This widget gets the lewis structure of chemical compounds.
There Are Three Lone Pairs On Each Fluorine Atom, And Two Lone Pairs On The Oxygen Atom.
Sketch the skeletal diagram of the molecule in. Calculate the number of valence electrons: Covalent bonds are formed when one electron from each atom forms an electron pair. Web 6 steps to draw the lewis structure of of2 step #1:
Web The Of2 Lewis Structure Consist Of One Oxygen Atom As The Central Atom And Two Fluorine Atom Are Present On The Either Side Of The Oxygen Atom.
Counting valence electrons begin by counting the total valence electrons in the molecule. Two (a pair of) valence electrons that are not used to form a covalent bond Web chemistry questions and answers. [ select] b) how many lone pairs of electrons are present on the central atom in the lewis structure?
This Widget Gets The Lewis Structure Of Chemical Compounds.
Since hydrogen is in group i it has one (1) valence electron in its shell. Web we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. Step 2 tells how many electrons are needed and step 1 is how many electrons you have. Web the lewis structure of of2 contains two single bonds, with oxygen in the center, and two fluorines on either side.
It Is A Chemical Formula For Oxygen Difluoride.
Draw lewis structures depicting the bonding in simple molecules. 8 + (6 × 7) = 50. If we look at the pauling. In this case, we can condense the last few steps, since not all of them apply.