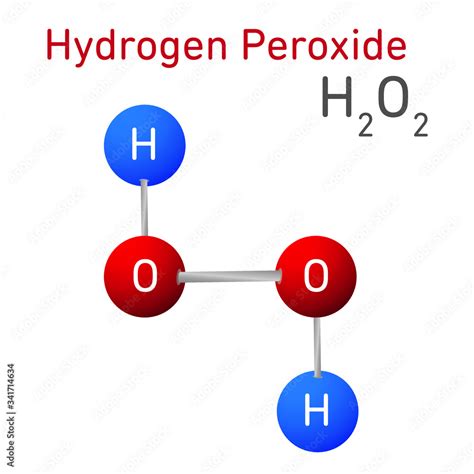
Hydrogen peroxide, commonly referred to as H2O2, is a chemical compound that has been widely used for various purposes due to its unique properties. It is a clear, colorless, and slightly viscous liquid that is highly soluble in water. The chemical formula of hydrogen peroxide is H2O2, indicating that it consists of two hydrogen atoms and two oxygen atoms. This compound is often confused with water (H2O) due to their similar molecular structures, but the additional oxygen atom in hydrogen peroxide gives it distinct characteristics and applications.
History and Discovery
Hydrogen peroxide has been known since the early 19th century, with its discovery often attributed to the French chemist Louis-Jacques Thenard in 1818. Thenard discovered that barium peroxide reacts with nitric acid to produce hydrogen peroxide. Initially, its production was challenging due to its instability, but over the years, more efficient methods have been developed, making it widely available for industrial, medical, and household uses.
Properties and Uses
Hydrogen peroxide is characterized by its strong oxidizing properties, which make it useful for a wide range of applications:
Bleaching Agent: One of the most common uses of hydrogen peroxide is as a bleaching agent. It is used in the paper industry to bleach wood pulp, in the textile industry for bleaching fabrics, and in hair care products for lightening hair color.
Disinfectant: Due to its ability to kill bacteria, viruses, and fungi, hydrogen peroxide is used as a disinfectant in medical settings, households, and for water treatment. It is especially effective against a broad spectrum of microorganisms, making it a preferred choice for cleaning surfaces and equipment.
Medical Applications: In medicine, hydrogen peroxide is used for wound cleaning and as an antiseptic. However, its use on wounds has become controversial due to concerns that it may damage tissue and delay healing. It is also used in dentistry for oral hygiene and as a mouthwash.
Food Industry: Hydrogen peroxide is used in the food industry as a sanitizer and as part of the processing to extend the shelf life of packaged foods.
Environmental Applications: It is used in the treatment of sewage and industrial waste, helping to break down organic matter.
Rocketry: High-concentration hydrogen peroxide (typically 90% or higher) is used as a monopropellant in some rocket engines due to its ability to decompose into steam and oxygen, producing thrust.
Safety and Handling
Despite its usefulness, hydrogen peroxide can be hazardous. Concentrated solutions are highly corrosive and can cause severe burns upon contact with skin. Ingestion can lead to gastrointestinal irritation and, in severe cases, gastric perforation. Inhalation of the vapors can cause respiratory irritation. Therefore, handling hydrogen peroxide requires caution, and it is essential to follow safety guidelines, including wearing protective clothing and ensuring good ventilation.
Conclusion
Hydrogen peroxide is a versatile compound with a wide array of applications due to its strong oxidizing properties. Its uses span from industrial processes to household cleaning and medical treatments. However, its handling requires caution due to its potential hazards. As research continues, new and safer methods of production and application are being developed, ensuring that hydrogen peroxide remains a valuable resource across various industries.
What are the primary uses of hydrogen peroxide?
+Hydrogen peroxide is primarily used as a bleaching agent, disinfectant, and in medical applications. It is also utilized in the food industry, environmental applications, and as a propellant in rocketry.
Is hydrogen peroxide safe for skin contact?
+No, concentrated hydrogen peroxide solutions are highly corrosive and can cause severe burns upon contact with skin. Lower concentrations, typically found in over-the-counter products, are safer but should still be used with caution.
What precautions should be taken when handling hydrogen peroxide?
+When handling hydrogen peroxide, it is essential to wear protective clothing, including gloves and goggles. Ensure good ventilation to avoid inhaling vapors, and follow the recommended dilution ratios for safe application.